Adult neurogenesis in the ventral hippocampus decreased among animal models of neurodevelopmental disorders
- PMID: 39764171
- PMCID: PMC11700985
- DOI: 10.3389/fncir.2024.1504191
Adult neurogenesis in the ventral hippocampus decreased among animal models of neurodevelopmental disorders
Abstract
Introduction: Autism spectrum disorder (ASD) is a neurodevelopmental condition characterized by deficits in social interaction and communication, along with restricted and repetitive behaviors. Both genetic and environmental factors contribute to ASD, with prenatal exposure to valproic acid (VPA) and nicotine being linked to increased risk. Impaired adult hippocampal neurogenesis, particularly in the ventral region, is thought to play a role in the social deficits observed in ASD.
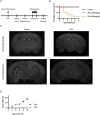
Methods: In this study, we investigated social behavior and adult hippocampal neurogenesis in C57BL/6J mice prenatally exposed to VPA or nicotine, as well as in genetically modified ASD models, including IQSEC2 knockout (KO) and NLGN3-R451C knock-in (KI) mice. Sociability and social novelty preference were evaluated using a three-chamber social interaction test. Adult hippocampal neurogenesis was assessed by BrdU and DCX immunofluorescence to identify newborn and immature neurons.
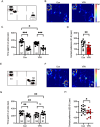
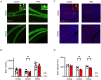
Results: VPA-exposed mice displayed significant deficits in social interaction, while nicotine-exposed mice exhibited mild impairment in social novelty preference. Both IQSEC2 KO and NLGN3-R451C KI mice demonstrated reduced adult neurogenesis, particularly in the ventral hippocampus, a region associated with social behavior and emotion. Across all ASD mouse models, a significant reduction in BrdU+/NeuN+ cells in the ventral hippocampus was observed, while dorsal hippocampal neurogenesis remained relatively unaffected. Similar reductions in DCX-positive cells were identified in VPA, nicotine, and NLGN3-R451C KI mice, indicating impaired proliferation or differentiation of neuronal progenitors.
Discussion: These findings suggest that impaired adult neurogenesis in the ventral hippocampus is a common hallmark across ASD mouse models and may underlie social behavior deficits. This study provides insight into region-specific neurogenic alterations linked to ASD pathophysiology and highlights potential targets for therapeutic interventions.
Keywords: IQSEC2; adult hippocampal neurogenesis; autism spectrum disorder; neuroligin 3; prenatal nicotine exposure; valproic acid.
Copyright © 2024 Sun, Ohashi, Mori, Mizuno, Zang, Guo, Kouyama-Suzuki, Shirai and Tabuchi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



Similar articles
-
Hippocampal Morphological Alterations and Oxidative Stress in Autism Spectrum Disorder Model Induced by Prenatal Exposure to Valproic Acid in Male and Female Mice.Hippocampus. 2025 Jul;35(4):e70024. doi: 10.1002/hipo.70024. Hippocampus. 2025. PMID: 40626515
-
Transplantation of mesenchymal stem cells reverses behavioural deficits and impaired neurogenesis caused by prenatal exposure to valproic acid.Oncotarget. 2017 Mar 14;8(11):17443-17452. doi: 10.18632/oncotarget.15245. Oncotarget. 2017. PMID: 28407680 Free PMC article.
-
Exploring the role of Cav3.2 calcium channels in autism-like cognitive behavior induced by prenatal valproic acid exposure.Neuroscience. 2025 Jun 21;577:71-79. doi: 10.1016/j.neuroscience.2025.05.011. Epub 2025 May 6. Neuroscience. 2025. PMID: 40339900
-
Prenatal valproate in rodents as a tool to understand the neural underpinnings of social dysfunctions in autism spectrum disorder.Neuropharmacology. 2019 Nov 15;159:107477. doi: 10.1016/j.neuropharm.2018.12.024. Epub 2019 Jan 9. Neuropharmacology. 2019. PMID: 30639388 Review.
-
Valproate-Induced Model of Autism in Adult Zebrafish: A Systematic Review.Cells. 2025 Jan 13;14(2):109. doi: 10.3390/cells14020109. Cells. 2025. PMID: 39851536 Free PMC article.
Cited by
-
The Competitive Loss of Cerebellar Granule and Purkinje Cells Driven by X-Linked Mosaicism in a Female Mouse Model of CASK-Related Disorders.Cells. 2025 May 17;14(10):735. doi: 10.3390/cells14100735. Cells. 2025. PMID: 40422238 Free PMC article.
References
-
- Alkam T., Kim H. C., Hiramatsu M., Mamiya T., Aoyama Y., Nitta A., et al. . (2013). Evaluation of emotional behaviors in young offspring of C57BL/6J mice after gestational and/or perinatal exposure to nicotine in six different time-windows. Behav. Brain Res. 239, 80–89. doi: 10.1016/j.bbr.2012.10.058, PMID: - DOI - PubMed
-
- Ávila-Gámiz F., Pérez-Cano A. M., Pérez-Berlanga J. M., Mullor-Vigo R. M., Zambrana-Infantes E. N., Santín L. J., et al. . (2023). Sequential treadmill exercise and cognitive training synergistically increase adult hippocampal neurogenesis in mice. Physiol. Behav. 266:114184. doi: 10.1016/j.physbeh.2023.114184, PMID: - DOI - PubMed
-
- Badawi M., Mori T., Kurihara T., Yoshizawa T., Nohara K., Kouyama-Suzuki E., et al. . (2021). Risperidone mitigates enhanced excitatory neuronal function and repetitive behavior caused by an ASD-associated mutation of SIK1. Front. Mol. Neurosci. 14:706494. doi: 10.3389/fnmol.2021.706494, PMID: - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

