Bioactive Materials Facilitate the Restoration of Neurological Function Post Cerebral Ischemic Stroke
- PMID: 39764188
- PMCID: PMC11701096
- DOI: 10.2147/IJN.S493987
Bioactive Materials Facilitate the Restoration of Neurological Function Post Cerebral Ischemic Stroke
Abstract
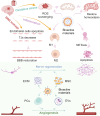
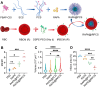
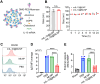
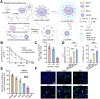
The recovery process following ischemic stroke is a complex undertaking involving intricate cellular and molecular interactions. Cellular dysfunction or aberrant pathways can lead to complications such as brain edema, hemorrhagic transformation, and glial scar hyperplasia, hindering angiogenesis and nerve regeneration. These abnormalities may contribute to long-term disability post-stroke, imposing significant burdens on both families and society. Current clinical interventions primarily focus on endovascular therapy, overlooking the protection of brain cells themselves. However, the use of bioactive materials in stroke management has shown notable safety and efficacy. By precisely targeting the ischemic site at a cellular and molecular level, this therapeutic approach mitigates ischemia-induced brain tissue damage and promotes site repair. This review examines the protective benefits of bioactive materials in reducing cell damage and facilitating nerve restoration in accordance with the pathophysiological basis of ischemic stroke. Enhanced understanding of ischemic stroke mechanisms has the potential to advance the targeted and efficient clinical use of bioactive materials.
Keywords: angiogenesis; bioactive materials; inflammation; ischemic stroke; nerve regeneration; oxidative stress.
© 2024 Wang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

