Wooden breast myopathy is characterized by satellite cell dysfunction and syndecan-4 shedding
- PMID: 39764382
- PMCID: PMC11701147
- DOI: 10.3389/fphys.2024.1513311
Wooden breast myopathy is characterized by satellite cell dysfunction and syndecan-4 shedding
Abstract
Introduction: Skeletal muscle satellite cells (MuSCs or stem cells) play a crucial role in muscle development, maintenance, and regeneration, supporting both hypertrophy and regenerative myogenesis. Syndecans (SDCs) act as communication bridges within the muscle microenvironment, regulating interactions with extracellular matrix components and contributing significantly to tissue repair and inflammation. Specifically, syndecan-4 (SDC4) is involved in muscle regeneration at multiple stages.
Methods: This study delves into the emerging challenge of wooden breast (WB) myopathy and its connection with SDC4. Our hypothesis proposes that disruptions in MuSC dynamics through SDC4 contribute to the increased incidence of breast myopathies observed in growing broilers. To test our hypothesis, non-affected and affected broilers were systematically selected, and the characteristics of WB myopathy were studied both in vitro and in vivo. SDC4 overexpression in MuSCs and blocking peptides (BPs) corresponding to the SDC4 ectodomain were used for investigating the role of SDC4 in muscle development and its shedding levels.
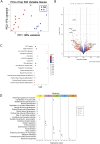
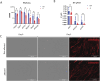
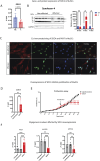
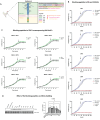
Results and discussion: In vivo examination of affected muscles revealed smaller fibers and changes in metabolic pathways. In vitro studies unveiled disrupted proliferation of MuSCs in WB myopathy, accompanied by the downregulation of several muscle markers. Investigation of the potential role of SDC4 in the pathogenesis of WB myopathy revealed a decreased tendency in SDC4 gene expression and increased shedding of its ectodomain. Moreover, we showed that SDC4 overexpression is linked to reduced proliferation in MuSCs and affected myogenesis. We detected an impaired proliferation of WB-affected MuSCs, revealing critical insights into the dysfunctional state of these cells in myopathy. Additionally, by treating MuSCs with blocking peptides derived from the SDC4 ectodomain, we identified altered proliferation. Taken together, this work contributes with valuable knowledge on the molecular mechanisms underlying WB myopathy and the role of SDC4 in this chicken myopathy.
Keywords: broiler chicken; myopathy; skeletal muscle satellite cells; syndecan-4; syndecans; wooden breast.
Copyright © 2024 Pejšková, Pisconti, Lunde, Ho, Solberg, Koga, Tengstrand, Carlson, Pedersen and Rønning.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Dysfunctional terminal differentiation of satellite cells in the wooden breast muscle of Ross 308 broiler chickens.Poult Sci. 2025 May;104(5):105041. doi: 10.1016/j.psj.2025.105041. Epub 2025 Mar 15. Poult Sci. 2025. PMID: 40121757 Free PMC article.
-
Characterization of wooden breast myopathy: a focus on syndecans and ECM remodeling.Front Physiol. 2023 Dec 5;14:1301804. doi: 10.3389/fphys.2023.1301804. eCollection 2023. Front Physiol. 2023. PMID: 38130476 Free PMC article.
-
Characterization of Pectoralis Major Muscle Satellite Cell Population Heterogeneity, Macrophage Density, and Collagen Infiltration in Broiler Chickens Affected by Wooden Breast.Front Physiol. 2020 May 27;11:529. doi: 10.3389/fphys.2020.00529. eCollection 2020. Front Physiol. 2020. PMID: 32536877 Free PMC article.
-
Skeletal muscle metabolic characteristics and fresh meat quality defects associated with wooden breast.Front Physiol. 2024 Oct 30;15:1501362. doi: 10.3389/fphys.2024.1501362. eCollection 2024. Front Physiol. 2024. PMID: 39539953 Free PMC article. Review.
-
Transcriptional regulation of autophagy in skeletal muscle stem cells.Dis Model Mech. 2025 Feb 1;18(2):DMM052007. doi: 10.1242/dmm.052007. Epub 2025 Feb 10. Dis Model Mech. 2025. PMID: 39925192 Free PMC article. Review.
Cited by
-
Molecular characterization of the heterogeneity of satellite cell populations isolated from an individual Turkey pectoralis major muscle.Front Physiol. 2025 Feb 20;16:1547188. doi: 10.3389/fphys.2025.1547188. eCollection 2025. Front Physiol. 2025. PMID: 40052144 Free PMC article.
-
Cardiac implications of chicken wooden breast myopathy.Front Physiol. 2025 Mar 5;16:1547661. doi: 10.3389/fphys.2025.1547661. eCollection 2025. Front Physiol. 2025. PMID: 40110183 Free PMC article.
References
-
- Andrews S. (2010). “FastQC: a quality control tool for high throughput sequence data,” in Babraham bioinformatics. Cambridge, United Kingdom: Babraham Institute.
-
- Andrews S. (2023). FastQC version 0.12.1. Babraham Bioinforma. Available at: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
LinkOut - more resources
Full Text Sources

