Platelets as crucial players in the dynamic interplay of inflammation, immunity, and cancer: unveiling new strategies for cancer prevention
- PMID: 39764464
- PMCID: PMC11701038
- DOI: 10.3389/fphar.2024.1520488
Platelets as crucial players in the dynamic interplay of inflammation, immunity, and cancer: unveiling new strategies for cancer prevention
Abstract
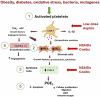
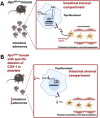
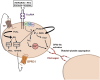
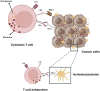
Inflammation plays a critical role in the pathogenesis of various diseases by promoting the acquisition of new functional traits by different cell types. Shared risk factors between cardiovascular disease and cancer, including smoking, obesity, diabetes, high-fat diet, low physical activity, and alcohol consumption, contribute to inflammation linked to platelet activation. Platelets contribute to an inflammatory state by activating various normal cells, such as fibroblasts, immune cells, and vascular cells. This activation is achieved by releasing diverse molecules from platelets, including lipids (eicosanoids), growth and angiogenic factors, and extracellular vesicles (EVs) rich in various RNA species. Antiplatelet agents like low-dose aspirin can prevent cardiovascular disease and cancer by inhibiting platelet functions beyond the antithrombotic action. Throughout the initial phases of tumorigenesis, the activation of platelets induces the overexpression of cyclooxygenase (COX)-2 in stromal cells, leading to increased biosynthesis of prostaglandin (PG)E2. This prostanoid can contribute to tumor development by inhibiting apoptosis, promoting cancer cell proliferation and migration, and immune evasion. Notably, platelets induce the epithelial-mesenchymal transition (EMT) in tumor cells, enhancing their metastatic potential. Two platelet eicosanoids, PGE2 (generated as a minor product of COX-1) and 12S-hydroxyeicosatetraenoic acid (HETE) [derived from the platelet-type 12-lipoxygenase (LOX)], contribute to EMT. In addition to the pharmacological inhibition of eicosanoid biosynthesis, a potential strategy for mitigating platelet-induced metastasis might encompass the inhibition of direct interactions between platelets and cancer cells. For example, there is promise in utilizing revacept to inhibit the interaction between platelet collagen receptors (particularly GPVI) and galectin-3 in cancer cells. Identifying these novel platelet functions suggests the potential application of antiplatelet agents, such as low-dose aspirin, in mitigating cancer risk, particularly in the case of colorectal cancer. It is necessary to investigate the effectiveness of other antiplatelet drugs, such as ADP P2Y12 receptor antagonists, in cancer prevention. Other new antiplatelet drugs, such as revacept and selective 12-LOX inhibitors, currently under clinical development, are of interest due to their low risk of bleeding. Platelets and EVs carry important clinical information because they contain specific proteins and RNAs associated with disease conditions. Their analysis can improve the accuracy of liquid biopsies for early cancer detection, monitoring progression, and assessing drug response.
Keywords: 12-lipoxygenase; antiplatelet agents; aspirin; cancer; extracellular vesicles; inflammation; platelets; revacept.
Copyright © 2024 Contursi, Tacconelli, Di Berardino, De Michele and Patrignani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
-
- Adili B. E. T., Mast K., Yeung J., Freedman J. C., Green A., Luci D. K., et al. (2017). First selective 12-LOX inhibitor, ML355, impairs thrombus formation and vessel occlusion in vivo with minimal effects on hemostasis. Arterioscler. Thromb. Vasc. Biol. 37, 1828–1839. 10.1161/ATVBAHA.117.309868 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

