SPI1 facilitates microfracture-mediated cartilage regeneration in the elderly by enhancing bone marrow stromal cells ctemness
- PMID: 39764517
- PMCID: PMC11700421
- DOI: 10.1177/20417314241311073
SPI1 facilitates microfracture-mediated cartilage regeneration in the elderly by enhancing bone marrow stromal cells ctemness
Abstract
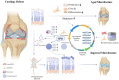
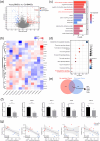
Bone marrow stimulation treatment by bone marrow stromal cells (BMSCs) released from the bone medullary cavity and differentiated into cartilage via microfracture surgery is a frequently employed technique for treating articular cartilage injuries, yet the treatment presents a main drawback of poor cartilage regeneration in the elderly. Prior research indicated that aging could decrease the stemness capacity of BMSCs, thus we made a hypothesis that increasing old BMSCs (OBMSCs) stemness might improve the results of microfracture in the elderly. First, we investigated the correlation between microfracture outcomes and BMSCs stemness using clinical data and animal experiments. The outcomes of microfracture surgery in the elderly were significantly decreased as compared with the young counterparts while the stemness capacity of OBMSCs was also significantly decreased, and they were positively correlated. To investigate the role of BMSCs stemness in microfracture, we developed microfracture-mimic cartilage regeneration organoid models. In vitro experiments identified SPI1 as a potential stemness target gene, which could enhance the stemness and chondrogenesis of OBMSCs. The implantation of cartilage regeneration organoids made by SPI1-overexpressed OBMSCs could notably enhance cartilage regeneration in the old rats as compared with the microfracture treatment alone. Furthermore, molecular docking suggested a possible interaction between SPI1 and 5-Aza-2'-deoxycytidine (5Aza). The application of 5Aza could significantly improve the result of microfracture by upregulating SPI1. In summary, we identified SPI1 as a novel stemness target of OBMSCs, which was beneficial for the improvement of microfracture-stimulated cartilage regeneration in the elderly.
Keywords: Microfracture; aging; bone marrow stromal cells; engineered organoids; stemness.
© The Author(s) 2025.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures





References
-
- Muthu S, Korpershoek JV, Novais EJ, et al. Failure of cartilage regeneration: emerging hypotheses and related therapeutic strategies. Nat Rev Rheumatol 2023; 19: 403–416. - PubMed
-
- Valderrabano V, Miska M, Leumann A, et al. Reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am J Sports Med 2013; 41: 519–527. - PubMed
-
- McIlwraith CW, Frisbie DD, Rodkey WG, et al. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy 2011; 27: 1552–1561. - PubMed
LinkOut - more resources
Full Text Sources

