Case report: Toxic epidermal necrolysis induced by tislelizumab in a patient with esophageal squamous cell carcinoma
- PMID: 39764552
- PMCID: PMC11700972
- DOI: 10.3389/fmed.2024.1522525
Case report: Toxic epidermal necrolysis induced by tislelizumab in a patient with esophageal squamous cell carcinoma
Abstract
Background: Immune checkpoint inhibitors (ICIs) have been widely applicated for the treatment of patients with advanced esophageal cancer. Skin-related adverse reactions are frequent with ICIs, with toxic epidermal necrolysis (TEN) being a severe and potentially life-threatening cutaneous reaction.
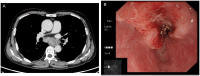
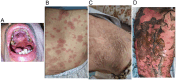
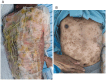
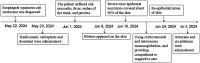
Case presentation: We present a case of a 70-year-old male with locally advanced esophageal cancer who developed severe toxic epidermal necrolysis (TEN) after 18 days of tislelizumab combined with chemotherapy. The condition rapidly progressed to cover approximately 90% of his body. After treatment with intravenous methylprednisolone, immunoglobulin, and antibiotics, along with active nutritional support and wound care, the patient recovered from TEN induced by tislelizumab.
Conclusion: Treatment for TEN is complex, and no standardized guidelines currently exist. We propose an economical, safe, effective, and simple strategy for similar TEN patients.
Keywords: esophageal squamous cell carcinoma; immune-related adverse events; skin toxicity; tislelizumab; toxic epidermal necrolysis.
Copyright © 2024 Wu, Xu and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

