PDPN+ cancer-associated fibroblasts enhance gastric cancer angiogenesis via AKT/NF-κB activation and the CCL2-ACKR1 axis
- PMID: 39764562
- PMCID: PMC11702504
- DOI: 10.1002/mco2.70037
PDPN+ cancer-associated fibroblasts enhance gastric cancer angiogenesis via AKT/NF-κB activation and the CCL2-ACKR1 axis
Abstract
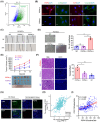
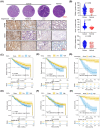
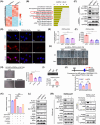
Cancer-associated fibroblasts (CAFs) are intrinsic components of the tumor microenvironment that promote cancer progression and metastasis. Through an unbiased integrated analysis of gastric tumor grade and stage, we identified a subset of proangiogenic CAFs characterized by high podoplanin (PDPN) expression, which are significantly enriched in metastatic lesions and secrete chemokine (CC-motif) ligand 2 (CCL2). Mechanistically, PDPN(+) CAFs enhance angiogenesis by activating the AKT/NF-κB signaling pathway. The canonical NF-κB signaling protein P65 binds to the promoter region of CCL2, inducing its expression. Additionally, we found that CCL2 interacts with its nonclassical receptor ACKR1 (expressed on endothelial cells) to exert its proangiogenic effects. Furthermore, the disruption of CCL2-ACKR1 communication via a CCL2 neutralizing antibody or the inhibition of AKT signaling transduction using AKT inhibitors effectively suppressed tumor growth. Together, this study elucidates the mechanism by which PDPN(+) CAFs promote angiogenesis, providing a deeper understanding of the molecular processes underlying CAF-mediated angiogenesis and suggesting potential therapeutic targets for gastric cancer treatment.
Keywords: CCL2; PDPN; angiogenesis; cancer‐associated fibroblasts; gastric cancer.
© 2025 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. - PubMed
-
- Shah MA. Update on metastatic gastric and esophageal cancers. J Clin Oncol. 2015;33(16):1760‐1769. - PubMed
-
- Claesson‐Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013;273(2):114‐127. - PubMed
-
- Kang Y‐K, Kang W, Di Bartolomeo M, et al. Randomized phase III ANGEL study of rivoceranib (apatinib)+ best supportive care (BSC) vs placebo+ BSC in patients with advanced/metastatic gastric cancer who failed≥ 2 prior chemotherapy regimens. Ann Oncol. 2019;30:v877‐v878.
LinkOut - more resources
Full Text Sources
