Effect of psilocybin versus escitalopram on depression symptom severity in patients with moderate-to-severe major depressive disorder: observational 6-month follow-up of a phase 2, double-blind, randomised, controlled trial
- PMID: 39764567
- PMCID: PMC11701471
- DOI: 10.1016/j.eclinm.2024.102799
Effect of psilocybin versus escitalopram on depression symptom severity in patients with moderate-to-severe major depressive disorder: observational 6-month follow-up of a phase 2, double-blind, randomised, controlled trial
Abstract
Background: Psilocybin therapy (PT) produces rapid and persistent antidepressant effects in major depressive disorder (MDD). However, the long-term effects of PT have never been compared with gold-standard treatments for MDD such as pharmacotherapy or psychotherapy alone or in combination.
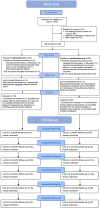
Methods: This is a 6-month follow-up study of a phase 2, double-blind, randomised, controlled trial involving patients with moderate-to-severe MDD. Participants were recruited from a hospital in the UK. Male or female patients with major depressive disorder (DSM-IV), moderate to severe depression (HAM-D ≥17), no MRI or SSRI contraindications, confirmed diagnosis by a GP or mental healthcare professional, aged 18-80, and competent in English were eligible. Patients were randomly assigned (1:1) to receive either two 25 mg doses of the psychedelic drug psilocybin administered orally combined with psychological support ('psilocybin therapy' or PT) and book-ended by further support or a 6-week course of the selective serotonin reuptake inhibitor (SSRI) escitalopram (administered daily at 10 mg for three weeks and 20 mg for the subsequent three weeks) plus matched psychological support ('escitalopram treatment' or ET). The primary outcome measure was change from baseline in the score on the 16-item Quick Inventory of Depressive Symptomatology-Self-Report (QIDS-SR-16) at week 6, which has been reported previously. Herein, we present results at the 6-month follow-up time point. Measures of social functioning, connectedness, and meaning in life constituted the study's secondary outcomes during follow-up. Safety in the follow-up period was not assessed. This trial is registered at ClinicalTrials.gov, NCT03429075.
Findings: Between January 15th, 2019 and March 20th, 2020, 59 patients were enrolled and 30 (11 females [37%] and 19 males [63%]) were assigned to the psilocybin group and 29 (9 females [31%] and 20 males [69%]) to the escitalopram group. 25 participants in the PT group and 21 in the ET group completed the 6-month follow-up. At the 6-month follow-up, both PT and ET conditions yielded sustained improvements in depressive symptom severity. The mean between-condition difference in QIDS-SR-16 scores at 6-months was 1.51 (95% CI: -1.35, 4.38; p = 0.311). Secondary outcomes demonstrated that PT had greater mean between-condition differences in functioning (WSAS: -7.46; 95% CI: -12.4, -2.47; p < 0.001), psychological connectedness (WCS: 11.02; 95% CI: 1.25, 20.83; p = 0.033), and meaning in life (MLQ: 4.86; 95% CI: 0.67, 9.05; p = 0.021) compared to ET.
Interpretation: Six-week intensive treatments with either psilocybin or escitalopram (with psychological support) for MDD were associated with long-term improvements in depressive symptom severity. The greater degree of improvement in the PT arm at follow-up on psychosocial functioning, meaning in life, and psychological connectedness suggests warrant future research. However, these results are descriptive and should be interpreted with caution. Key limitations of the study include its suboptimal power to detect small but meaningful differences between treatments, missing data, the potential use of additional interventions during the follow-up period, and reliance on self-reported treatment assessments. These factors may affect the interpretation of the study findings and should be considered when evaluating the results.
Funding: The Alexander Mosley Charitable Trust and by the founding partners of Imperial College London's Centre for Psychedelic Research.
Keywords: Depression; Follow-up; Psilocybin; SSRIs.
© 2024 The Authors.
Conflict of interest statement
RCH is a scientific advisor to Mindstate Design Lab. DE is a paid advisor for Aya Biosciences, Clerkenwell Health, Lophora Aps, and Mindstate Design Lab and had a role on Data Safety Monitoring Board on SmallPharma Ltd DMT depression trial. DJN has received consulting fees from Algernon and H. Lundbeck and Beckley Psytech, advisory board fees from COMPASS Pathways and lecture fees from Takeda and Otsuka and Janssen plus owns stock in Alcarelle, Awakn, and Psyched Wellness. B.W. owns Axial Therapeutic Research, Inc., a company investigating the safety and effectiveness of alternative treatments for military veteran health. BG and MBJ have received consulting fees from Cybin Inc. TB has received consulting fees from Adamo Biosciences. KTG received grants/contracts from Northern Ontario Academic Medicine Association: Clinical Innovation Opportunities Fund (2022/1–2023/1), Sir Mortimer B. Davis-Jewish General Hospital Foundation (The): JGH Québec Network on Suicide, Mood Disorders and Related Disorders. None of the other authors reported conflicts of interest. Compass Pathways delivered the study drug (psilocybin COMP360) and via contract agreement with Imperial Collge (study sponsor and IP holder) were granted access to safety data from the study.
Figures



References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. - DOI - PMC - PubMed
-
- Cosci F., Mansueto G., Fava G.A. Relapse prevention in recurrent major depressive disorder. A comparison of different treatment options based on clinical experience and a critical review of the literature. Int J Psychiatry Clin Pract. 2020;24:341–348. doi: 10.1080/13651501.2020.1779308. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

