Exploration of the feasibility of clinical application of phage treatment for multidrug-resistant Serratia marcescens-induced pulmonary infection
- PMID: 39764739
- PMCID: PMC11740298
- DOI: 10.1080/22221751.2025.2451048
Exploration of the feasibility of clinical application of phage treatment for multidrug-resistant Serratia marcescens-induced pulmonary infection
Abstract
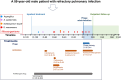
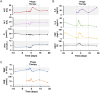
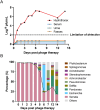
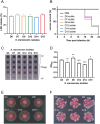
Serratia marcescens (S. marcescens) commonly induces refractory infection due to its multidrug-resistant nature. To date, there have been no reports on the application of phage treatment for S. marcescens infection. This study was conducted to explore the feasibility of phage application in treating refractory S. marcescens infection by collaborating with a 59-year-old male patient with a pulmonary infection of multidrug-resistant S. marcescens. Our experiments included three domains: i) selection of the appropriate phage, ii) verification of the efficacy and safety of the selected phage, iii) confirmation of phage-bacteria interactions. Our results showed that phage Spe5P4 is appropriate for S. marcescens infection. Treatment with phage Spe5P4 showed good efficacy, manifested as amelioration of symptoms, hydrothorax examinations, and chest computed tomography findings. Phage treatment did not worsen hepatic and renal function, immunity-related indices, or indices of routine blood examination. It did not induce or deteriorate drug resistance of the involved antibiotics. Importantly, no adverse events were reported during the treatment or follow-up periods. Thus, phage treatment showed satisfactory safety. Finally, we found that phage treatment did not increase the bacterial load, cytotoxicity, virulence, or phage resistance of S. marcescens, indicating satisfactory phage-bacteria interactions between Spe5P4 and S. marcescens, which are useful for the future application of phage Spe5P4 against S. marcescens. This work provides evidence and a working basis for further application of phage Spe5P4 in treating refractory S. marcescens infections. We also provided a methodological basis for investigating clinical application of phage treatment against multidrug-resistant bacterial infections in the future.
Keywords: Phage treatment; Serratia marcescens (S. marcescens); efficacy and safety; multidrug-resistance; phage Spe5P4.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
Cefepime shows good efficacy and no antibiotic resistance in pneumonia caused by Serratia marcescens and Proteus mirabilis - an observational study.BMC Pharmacol Toxicol. 2016 Mar 23;17:10. doi: 10.1186/s40360-016-0056-y. BMC Pharmacol Toxicol. 2016. PMID: 27004519 Free PMC article.
-
Genome evolution and plasticity of Serratia marcescens, an important multidrug-resistant nosocomial pathogen.Genome Biol Evol. 2014 Aug;6(8):2096-110. doi: 10.1093/gbe/evu160. Genome Biol Evol. 2014. PMID: 25070509 Free PMC article.
-
Proteomic profile of multidrug-resistant Serratia marcescens under meropenem challenge.Microb Pathog. 2025 Jul;204:107570. doi: 10.1016/j.micpath.2025.107570. Epub 2025 Apr 11. Microb Pathog. 2025. PMID: 40222567
-
Serratia marcescens.J Med Microbiol. 1997 Nov;46(11):903-12. doi: 10.1099/00222615-46-11-903. J Med Microbiol. 1997. PMID: 9368530 Review.
-
Serratia infections: from military experiments to current practice.Clin Microbiol Rev. 2011 Oct;24(4):755-91. doi: 10.1128/CMR.00017-11. Clin Microbiol Rev. 2011. PMID: 21976608 Free PMC article. Review.
Cited by
-
Exploring the Association of Antimicrobial Use with Serratia marcescens Resistance Rates via Multiple Linear Regression.Infect Drug Resist. 2025 Aug 13;18:4039-4051. doi: 10.2147/IDR.S527225. eCollection 2025. Infect Drug Resist. 2025. PMID: 40827268 Free PMC article.
References
-
- Guo Y, Zou G, Kerdsin A, et al. . Characterization of NMCR-3, NMCR-4 and NMCR-5, three novel non-mobile colistin resistance determinants: implications for MCR-3, MCR-7, and MCR-5 progenitors, respectively. Drug Resist Updat. 2024;75:101088. - PubMed
-
- d’Herelle F. An invisible microbe that is antagonistic to the dysentery bacillus. CR Acad Sci. 1917;165:373–375.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
