Comparison of Fecal Microbiota and Metabolites Between Captive and Grazing Male Reindeer
- PMID: 39765510
- PMCID: PMC11672574
- DOI: 10.3390/ani14243606
Comparison of Fecal Microbiota and Metabolites Between Captive and Grazing Male Reindeer
Abstract
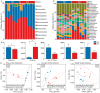
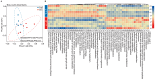
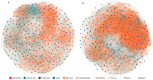
The reindeer (Rangifer tarandus) is a circumpolar member of the Cervidae family, and has adapted to a harsh environment. Summer is a critical period for reindeer, with peak digestibility facilitating body fat accumulation. The gut microbiota plays a pivotal role in nutrient metabolism, and is affected by captivity. However, differences in the composition of the gut microbiota and metabolites between captive and grazing reindeer during summer remain poorly understood. Here, we conducted a comparative study of the fecal microbiota and metabolites between captive (n = 6) and grazing (n = 6) male reindeer, using full-length 16S rRNA gene sequencing and gas chromatography-time-of-flight mass spectrometry, respectively. Our results indicated that Prevotella, Phocaeicola, Papillibacter, Muribaculum, and Bacteroides were the predominant genera in the feces of reindeer. However, microbial diversity was significantly higher in captive reindeer compared to their grazing counterparts. Principal coordinate analysis revealed significant differences in the fecal microbiota between captive and grazing reindeer. In captive reindeer, the relative abundances of the genera Clostridium, Paraprevotella, Alistipes, Paludibacter, Lentimicrobium, Paraclostridium, and Anaerovibrio were significantly higher, while those of the genera Prevotella, Phocaeicola, Pseudoflavonifractor, and Lactonifactor were significantly lower. A comparison of predicted functions indicated that pathways involved in fat digestion and absorption, histidine metabolism, lysine biosynthesis, and secondary bile acid biosynthesis were more abundant in captive reindeer, whereas the pathways of fructose and mannose metabolism and propanoate metabolism were less abundant. An untargeted metabolomic analysis revealed that 624 metabolites (e.g., amino acids, lipids, fatty acids, and bile acids) and 645 metabolites (e.g., carbohydrates and purines) were significantly increased in the feces of captive and grazing reindeer, respectively. In conclusion, we unveiled significant differences in fecal microbiota and metabolites between captive and grazing male reindeer, with the results suggesting a potentially enhanced ability to utilize plant fibers in grazing reindeer.
Keywords: captivity; full-length 16S rRNA gene; metabolome; reindeer; summer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Yildirim E., Ilina L., Laptev G., Filippova V., Brazhnik E., Dunyashev T., Dubrovin A., Novikova N., Tiurina D., Tarlavin N., et al. The structure and functional profile of ruminal microbiota in young and adult reindeers (Rangifer tarandus) consuming natural winter-spring and summer-autumn seasonal diets. PeerJ. 2021;9:e12389. doi: 10.7717/peerj.12389. - DOI - PMC - PubMed
-
- Pösö A. Seasonal changes in reindeer physiology. Rangifer. 2005;25:31–38. doi: 10.7557/2.25.1.335. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

