Delimitation of Endangered Telmatobius Species (Anura: Telmatobiidae) of the Chilean Salt Puna
- PMID: 39765516
- PMCID: PMC11672803
- DOI: 10.3390/ani14243612
Delimitation of Endangered Telmatobius Species (Anura: Telmatobiidae) of the Chilean Salt Puna
Abstract
Clarifying the taxonomic status and distribution of endangered species is crucial to their conservation. In this study, we contrasted different lines of evidence (morphology, mtDNA, and nucDNA: microsatellites and SNP) to clarify the taxonomic status of endangered Telmatobius water frog species and unidentified populations that inhabit the Salt Puna in Chile. We studied population differentiation and species divergence by performing morphometric, population genetic and species delimitation analyses. The results confirmed the species status of Telmatobius fronteriensis and T. philippii, as they exhibited morphometric, mitochondrial and genomic SNP divergence. Although Bayes factor delimitation analysis indicated that the Telmatobius populations of Ascotán and Carcote could represent a new species, their few mitochondrial differences and similar morphology with respect to T. philippii suggested otherwise. Instead, they can be considered an evolutionarily significant unit of T. philippii that has differentiated from the type locality. These results extend the geographic distribution of T. philippii, which is categorized as critically endangered by the IUCN.
Keywords: Andean Altiplano; Tauca paleolake; amphibians; species delimitation; taxonomy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
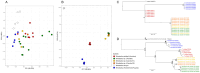

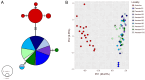
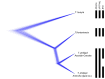

References
-
- Rannala B., Yang Z. Species Delimitation. In: Scornavacca C., Delsuc F., Galtier N., editors. Phylogenetics in the Genomic Era. No Commercial Publisher; London, UK: 2020. [(accessed on 17 September 2024)]. pp. 1–18. Chapter 5.5. Available online: https://hal.inria.fr/PGE.
-
- Stanton D.W., Frandsen P., Waples R.K., Heller R., Russo I.R.M., Orozco-terWengel P.A., Tingskov C.E., Siegismund H.R., Bruford M.W. More grist for the mill? Species delimitation in the genomic era and its implications for conservation. Conserv. Genet. 2019;20:101–113. doi: 10.1007/s10592-019-01149-5. - DOI
Grants and funding
- ANID FONDECYT Postdoctorado 3220183/Agencia Nacional de Investigación y Desarrollo
- ANID PIA/BASAL FB0002/Agencia Nacional de Investigación y Desarrollo
- ANID FONDECYT 1200419/Agencia Nacional de Investigación y Desarrollo
- ANID- FONDECYT 1221214/Agencia Nacional de Investigación y Desarrollo
- CHIC ANID FB210018/Agencia Nacional de Investigación y Desarrollo
LinkOut - more resources
Full Text Sources

