Parasite Screening in Wild Passerines: Enhancing Diagnostic Approaches in Wildlife Rehabilitation Centers
- PMID: 39765569
- PMCID: PMC11727621
- DOI: 10.3390/ani14243664
Parasite Screening in Wild Passerines: Enhancing Diagnostic Approaches in Wildlife Rehabilitation Centers
Abstract
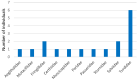
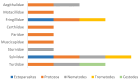
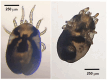
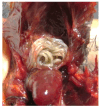
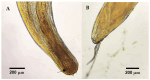
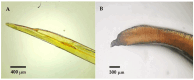
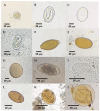
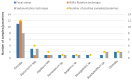
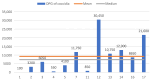
The order Passeriformes is the richest and most abundant group of birds, but despite numerous parasites being identified in wild birds, this order has received limited focus. This study analyzed 17 passerines admitted to the Grupo de Rehabilitación de la Fauna Autóctona y su Hábitat (GREFA), a wildlife rehabilitation center in Spain, during October to December 2022. Necropsies were conducted to determine the presence of parasites, and intestinal contents were analyzed using fecal smear, flotation and sedimentation techniques and the McMaster method. Sixteen passerines (94.1%) were positive for parasites. Identified species included Monojoubertia microhylla and the genera Ornithonyssus sp., Diplotriaena spp., Serratospiculum sp., Porrocaecum sp., Capillaria spp., Syngamus sp., Strongyloides sp. and Brachylecithum sp., besides cestodes and coccidia. The comparative analysis of parasitological diagnostic techniques showed that the Willis flotation technique was effective for detecting coccidia. However, to obtain more accurate results for other parasites, it is important to complement this technique with direct examination or sedimentation techniques. Among the 12 passerines positive for coccidia, oocyst counts per gram of intestinal contents ranged from 100 to 30,450, with a median of 7350. This study provides valuable insights into the parasitic fauna of Passeriformes, serving as a cornerstone for future research and enhancing our understanding of these avian species.
Keywords: Passeriformes; Spain; birds; coccidia; helminths; parasites; passerines; wild.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Atkinson C.T., Thomas N.J., Hunter D.B., editors. Parasitic Diseases of Wild Birds. 1st ed. Wiley-Blackwell; Ames, IA, USA: 2008. pp. 3–463.
-
- Carrera-Játiva P.D., Morgan E.R., Barrows M., Jiménez-Uzcátegui G., Armijos Tituaña J.R. Free-ranging avifauna as a source of generalist parasites for captive birds in zoological settings: An overview of parasite records and potential for cross-transmission. J. Adv. Vet. Anim. Res. 2020;7:482–500. - PMC - PubMed
-
- Sajid M., Ehsan N. Insect ectoparasites on wild migratory birds: A review. Anim. Sci. J. 2017;8:1–8.
-
- Krone O., Cooper J.E. Parasitic diseases in Birds of prey-Health & Disease. 3rd ed. Blackwell Science; Oxford, UK: 2002. pp. 105–120.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

