Brain-Periphery Axes: The Potential Role of Extracellular Vesicles-Delivered miRNAs
- PMID: 39765723
- PMCID: PMC11673379
- DOI: 10.3390/biology13121056
Brain-Periphery Axes: The Potential Role of Extracellular Vesicles-Delivered miRNAs
Abstract
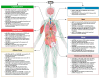
Bidirectional communication between the central nervous system (CNS) and peripheral organs and tissue has been widely documented in physiological and pathological conditions. This communication relies on the bilateral transmission of signaling molecules and substances that circulate throughout the body and reach their target site(s) via the blood and other biological fluids (e.g., the cerebrospinal fluid, the lymph). One of the mechanisms by which these molecular messengers are exchanged is through the secretion of extracellular vesicles (EVs). EVs are known to mediate cell-to-cell communication by delivering biological molecules, including nucleic acids, proteins, lipids, and various other bioactive regulators. Moreover, EVs can cross the blood-brain barrier (BBB), enabling direct communication between the periphery and the brain. In particular, the delivery of microRNAs (miRNAs) can modulate the expression profiles of recipient cells, thereby influencing their functions. This review synthesizes current findings about the brain-periphery cross-talk mediated by EVs-delivered miRNAs. Although this mechanism has been definitively shown in a few cases, much evidence indirectly indicates that it could mediate brain-peripherical organs/tissue communication, especially in pathological conditions. Therefore, understanding this process could provide valuable insights for the treatment and management of neurological and systemic diseases.
Keywords: adipose tissue; brain; brain–periphery bidirectional communication; extracellular vesicles-delivered miRNAs; gut; heart; immune system; kidney; lung; skeletal muscle.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

