Potential Opportunities for Pharmacogenetic-Based Therapeutic Exploitation of Xanthine Dehydrogenase in Cardiovascular Disease
- PMID: 39765766
- PMCID: PMC11672463
- DOI: 10.3390/antiox13121439
Potential Opportunities for Pharmacogenetic-Based Therapeutic Exploitation of Xanthine Dehydrogenase in Cardiovascular Disease
Abstract
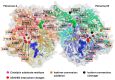
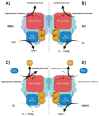
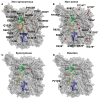
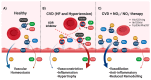
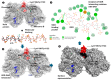
The majority of naturally occurring mutations of the human gene XDH, are associated with reduced or completely absent xanthine oxidoreductase (XOR) activity, leading to a disease known as classical xanthinuria, which is due to the accumulation and excretion of xanthine in urine. Three types of classical xanthinuria have been identified: type I, characterised by XOR deficiency, type II, caused by XOR and aldehyde oxidase (AO) deficiency, and type III due to XOR, AO, and sulphite oxidase (SO) deficiency. Type I and II are considered rare autosomal recessive disorders, a condition where two copies of the mutated gene must be present to develop the disease or trait. In most cases, xanthinuria type I and II result to be asymptomatic, and only occasionally lead to renal failure due to urolithiasis caused by xanthine deposition. However, in the last 10-15 years, new observations have been made about the link between naturally occurring mutations and pathological phenotypes particularly pertinent to cardiovascular diseases (CVD). These links have been attributed to a genetically driven increase of XOR expression and activity that is responsible for what is thought to be damaging uric acid (UA) and reactive oxygen species (ROS) accumulation, nitric oxide (·NO) depletion and endothelial dysfunction. In this review, we discuss the importance of genetics for interindividual variability of XOR expression and activity while focusing mainly on those variants thought to be relevant for CVD. In addition, we discuss the potential exploitation of the genetically driven increase of XOR activity to deliver more beneficial bioavailable ·NO. Finally, we examine the effect that non-synonymous mutations have on the tertiary structure of the protein and consequently on its capacity to interact with glycosaminoglycans (GAGs) localised on the outer surface of endothelial cells.
Keywords: NO; cardiovascular diseases; glycosaminoglycans; nitrite; nitrite reduction; xanthine oxidoreductase.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Amrita Ahluwalia is a Co-Director of Heartbeet Ltd. and IoNa Therapeutics seeking to identify therapeutic opportunities for dietary nitrate.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

