Exploring Oxidative Stress and Metabolic Dysregulation in Lung Tissues of Offspring Rats Exposed to Prenatal Polystyrene Microplastics: Effects of Melatonin Treatment
- PMID: 39765788
- PMCID: PMC11672973
- DOI: 10.3390/antiox13121459
Exploring Oxidative Stress and Metabolic Dysregulation in Lung Tissues of Offspring Rats Exposed to Prenatal Polystyrene Microplastics: Effects of Melatonin Treatment
Abstract
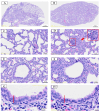
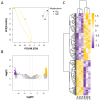
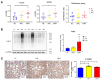
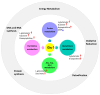
Metabolomics research provides a clearer understanding of an organism's metabolic state and enables a more accurate representation of its functional performance. This study aimed to investigate changes in the metabolome of lung tissues resulting from prenatal exposure to polystyrene microplastics (PS-MPs) and to understand the underlying mechanisms of lung damage in rat offspring. We conducted metabolomic analyses of lung tissue from seven-day-old rat pups exposed to prenatal PS-MPs. Our findings revealed that prenatal exposure to PS-MPs led to significantly increased oxidative stress in lung tissues, characterized by notable imbalances in nucleic acid metabolism and altered profiles of specific amino acids. Furthermore, we evaluated the therapeutic effects of melatonin treatment on lung function in 120-day-old offspring and found that melatonin treatment significantly improved lung function and histologic change in the affected offspring. This study provides valuable biological insights into the mechanisms underlying lung damage caused by prenatal PS-MPs exposure. Future studies should focus on validating the results of animal experiments in humans, exploring additional therapeutic mechanisms of melatonin, and developing suitable protocols for clinical use.
Keywords: lung; melatonin; metabolome; microplastics; offspring; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

