Erinacine A-Enriched Hericium erinaceus Mycelium Ethanol Extract Lessens Cellular Damage in Cell and Drosophila Models of Spinocerebellar Ataxia Type 3 by Improvement of Nrf2 Activation
- PMID: 39765823
- PMCID: PMC11673478
- DOI: 10.3390/antiox13121495
Erinacine A-Enriched Hericium erinaceus Mycelium Ethanol Extract Lessens Cellular Damage in Cell and Drosophila Models of Spinocerebellar Ataxia Type 3 by Improvement of Nrf2 Activation
Abstract
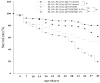
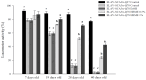
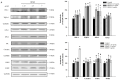
Spinocerebellar ataxia type 3 (SCA3), caused by the abnormal expansion of polyglutamine (polyQ) in the ataxin-3 protein, is one of the inherited polyQ neurodegenerative diseases that share similar genetic and molecular features. Mutant polyQ-expanded ataxin-3 protein is prone to aggregation in affected neurons and is predominantly degraded by autophagy, which is beneficial for neurodegenerative disease treatment. Not only does mutant polyQ-expanded ataxin-3 increase susceptibility to oxidative cytotoxicity, but it also hampers antioxidant potency in neuronal cells. Nuclear factor erythroid-derived 2-like 2 (Nrf2), a master transcription factor that controls antioxidant and detoxification gene expression, plays a crucial role in neuroprotection in SCA3 and other neurodegenerative diseases. The present data showed that treatment with erinacine A-enriched Hericium erinaceus mycelium ethanol extract (HEME) extended longevity and improved locomotor activity in ELAV-SCA3tr-Q78 transgenic Drosophila. Moreover, HEME treatment enhanced antioxidant potency and autophagy, which, in turn, corrected levels of mutant polyQ-expanded ataxin-3 and restrained protein aggregation in both cell and Drosophila models of SCA3. Markedly, HEME increased the activation of Nrf2. Silencing Nrf2 protein expression negated most of the promising effects of HEME on SK-N-SH-MJD78 cells, highlighting the critical role of increased Nrf2 activation in the efficacy of HEME treatment. These findings suggest that HEME has therapeutic potential in SCA3 by enhancing autophagic and Nrf2-mediated antioxidant pathways, which may also influence neurodegenerative progression in other polyQ diseases.
Keywords: Nrf2; PolyQ diseases; autophagy; erinacine A-enriched Hericium erinaceus mycelium; mutant polyQ-expanded ataxin-3; oxidative stress; spinocerebellar ataxia type 3.
Conflict of interest statement
Authors C.-C.C. and L.-Y.L. were employed by company Grape King Bio, Ltd. All other authors declare no competing interests.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

