Multitarget Effects of Nrf2 Signalling in the Brain: Common and Specific Functions in Different Cell Types
- PMID: 39765831
- PMCID: PMC11673142
- DOI: 10.3390/antiox13121502
Multitarget Effects of Nrf2 Signalling in the Brain: Common and Specific Functions in Different Cell Types
Abstract
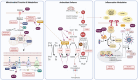
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a crucial regulator of cellular defence mechanisms, essential for maintaining the brain's health. Nrf2 supports mitochondrial function and protects against oxidative damage, which is vital for meeting the brain's substantial energy and antioxidant demands. Furthermore, Nrf2 modulates glial inflammatory responses, playing a pivotal role in preventing neuroinflammation. This review explores these multifaceted functions of Nrf2 within the central nervous system, focusing on its activity across various brain cell types, including neurons, astrocytes, microglia, and oligodendrocytes. Due to the brain's vulnerability to oxidative stress and metabolic challenges, Nrf2 is emerging as a key therapeutic target to enhance resilience against oxidative stress, inflammation, mitochondrial dysfunction, and demyelination, which are central to many neurodegenerative diseases.
Keywords: Nrf2; antioxidants; astrocytes; brain; inflammation; microglia; mitochondria; neurodegeneration; neurons.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Komatsu M., Kurokawa H., Waguri S., Taguchi K., Kobayashi A., Ichimura Y., Sou Y.S., Ueno I., Sakamoto A., Tong K.I., et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. - DOI - PubMed
-
- Salazar M., Rojo A.I., Velasco D., de Sagarra R.M., Cuadrado A. Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J. Biol. Chem. 2006;281:14841–14851. doi: 10.1074/jbc.M513737200. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

