Chitosan Nanoparticle-Mediated Delivery of Alstonia venenata R.Br. Root Methanolic Extract: A Promising Strategy for Breast Cancer Therapy in DMBA-Induced Breast Cancer in Sprague Dawley Rats
- PMID: 39765841
- PMCID: PMC11673636
- DOI: 10.3390/antiox13121513
Chitosan Nanoparticle-Mediated Delivery of Alstonia venenata R.Br. Root Methanolic Extract: A Promising Strategy for Breast Cancer Therapy in DMBA-Induced Breast Cancer in Sprague Dawley Rats
Abstract
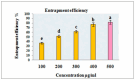
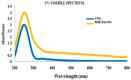
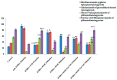
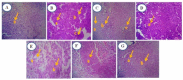
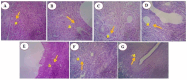
Alstonia venenata R.Br., a plant native to the Western Ghats, is recognized for its diverse medicinal properties. The plant's extracts, particularly rich in alkaloids and other bioactive compounds, have shown potential anticancer activity. This study investigates the therapeutic potential of chitosan nanoparticles (CNPs) loaded with the root methanolic extract (RME) of A. venenata in combating breast cancer induced by dimethylbenz(a)anthracene (DMBA) in female Sprague Dawley rats. The RME-loaded chitosan nanoparticles (RME-EnCNPs) were synthesized and characterized, and their in vivo efficacy was evaluated. Treatment with RME-EnCNPs significantly inhibited tumor progression, which is evidenced by reduced tumor volume, burden, and incidence. Moreover, the nanoparticles demonstrated a sustained release of the active compounds, leading to marked improvements in various biochemical, enzymatic, and histopathological parameters. The study found that both RME and RME-EnCNPs effectively suppressed tumor growth, with RME-EnCNPs showing superior efficacy in modulating tumor progression. Antioxidant assays revealed that treatment with RME-EnCNPs (500 mg/kg) resulted in significant increases in total protein, superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), and glutathione (GSH) levels, alongside a marked reduction in lipid peroxidation (LPO) (p < 0.001). These findings suggest that RME-EnCNPs exert a potent antioxidant effect, mitigating oxidative stress within the tumor microenvironment. The root extract of A. venenata and its nanoparticle formulation hold promise as a potential therapeutic agent for breast cancer, warranting further investigation to isolate active bioactive compounds and elucidate their mechanisms of action.
Keywords: Alstonia venenata; anticancer activity; antioxidant activity; chitosan nanoparticles; dimethylbenz(a)anthracene; rats.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










References
-
- Ferlay J., Colombet M., Soerjomataram I., Parkin D.M., Piñeros M., Znaor A., Bray F., Global Cancer Observatory: Cancer Today [Internet] Lyon: International Agency for Research on Cancer. 2018. [(accessed on 1 June 2022)]. Available online: https://gco.iarc.fr/today/
Grants and funding
LinkOut - more resources
Full Text Sources

