Striking Cardioprotective Effects of an Adiponectin Receptor Agonist in an Aged Mouse Model of Duchenne Muscular Dystrophy
- PMID: 39765879
- PMCID: PMC11674016
- DOI: 10.3390/antiox13121551
Striking Cardioprotective Effects of an Adiponectin Receptor Agonist in an Aged Mouse Model of Duchenne Muscular Dystrophy
Abstract
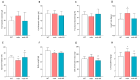
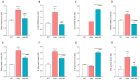
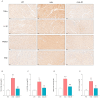
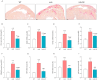
Adiponectin (ApN) is a hormone with potent effects on various tissues. We previously demonstrated its ability to counteract Duchenne muscular dystrophy (DMD), a severe muscle disorder. However, its therapeutic use is limited. AdipoRon, an orally active ApN mimic, offers a promising alternative. While cardiomyopathy is the primary cause of mortality in DMD, the effects of ApN or AdipoRon on dystrophic hearts have not been investigated. Our recent findings demonstrated the significant protective effects of AdipoRon on dystrophic skeletal muscle. In this study, we investigated whether AdipoRon effects could be extended to dystrophic hearts. As cardiomyopathy develops late in mdx mice (DMD mouse model), 14-month-old mdx mice were orally treated for two months with AdipoRon at a dose of 50 mg/kg/day and then compared with untreated mdx and wild-type (WT) controls. Echocardiography revealed cardiac dysfunction and ventricular hypertrophy in mdx mice, which were fully reversed in AdipoRon-treated mice. AdipoRon also reduced markers of cardiac inflammation, oxidative stress, hypertrophy, and fibrosis while enhancing mitochondrial biogenesis via ApN receptor-1 and CAMKK2/AMPK pathways. Remarkably, treated mice also showed improved skeletal muscle strength and endurance. By offering protection to both cardiac and skeletal muscles, AdipoRon holds potential as a comprehensive therapeutic strategy for better managing DMD.
Keywords: AMPK; Duchenne muscular dystrophy; adiponectin; cardiomyopathy; fibrosis; inflammation; mitochondria.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

