Antioxidant, Anti-Inflammatory, and Anticancer Activities of Five Citrus Peel Essential Oils
- PMID: 39765890
- PMCID: PMC11672981
- DOI: 10.3390/antiox13121562
Antioxidant, Anti-Inflammatory, and Anticancer Activities of Five Citrus Peel Essential Oils
Abstract
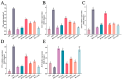
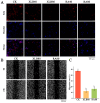
Citrus peel essential oil (CPEO) is favored by people for its aromatic scent, while also possessing numerous bioactive compounds that are advantageous to human health. This study evaluated the antioxidant, anti-inflammatory, and anticancer activities of CPEOs through cell experiments. The results showed that CPEOs could increase the activity of the antioxidant enzyme system and nonenzymatic defence system in H2O2-treated RAW 264.7 cells by reducing cellular lipid peroxidation. CPEOs also reduced the nitric oxide production induced by lipopolysaccharide treatment in RAW 264.7 cells while decreasing proinflammatory cytokines expression and increasing anti-inflammatory cytokine expression. Wound healing assays, flow cytometry, and quantitative real-time fluorescent quantitative PCR (qRT-PCR) revealed that CPEOs could induce apoptosis in U87 cells through the mitochondrial apoptotic pathway. These findings indicate that CPEOs possess excellent antioxidant, anti-inflammatory, and anticancer activity potential, making them suitable for use in functional antioxidant and anti-inflammatory foods and nutritional health products.
Keywords: anti-inflammatory; anticancer; antioxidants; citrus; essential oil.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Suri S., Singh A., Nema P.K. Current Applications of Citrus Fruit Processing Waste: A Scientific Outlook. Appl. Food Res. 2022;2:100050. doi: 10.1016/j.afres.2022.100050. - DOI
-
- Ben Hsouna A., Sadaka C., Generalić Mekinić I., Garzoli S., Švarc-Gajić J., Rodrigues F., Morais S., Moreira M.M., Ferreira E., Spigno G., et al. The Chemical Variability, Nutraceutical Value, and Food-Industry and Cosmetic Applications of Citrus Plants: A Critical Review. Antioxidants. 2023;12:481. doi: 10.3390/antiox12020481. - DOI - PMC - PubMed
-
- Palma C.E., Cruz P.S., Cruz D.T.C., Bugayong A.M.S., Castillo A.L. Chemical Composition and Cytotoxicity of Philippine Calamansi Essential Oil. Ind. Crop. Prod. 2019;128:108–114. doi: 10.1016/j.indcrop.2018.11.010. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

