Neuroprotective Potential of Indole-Based Compounds: A Biochemical Study on Antioxidant Properties and Amyloid Disaggregation in Neuroblastoma Cells
- PMID: 39765912
- PMCID: PMC11673510
- DOI: 10.3390/antiox13121585
Neuroprotective Potential of Indole-Based Compounds: A Biochemical Study on Antioxidant Properties and Amyloid Disaggregation in Neuroblastoma Cells
Abstract
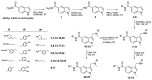
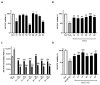
Based on the established neuroprotective properties of indole-based compounds and their significant potential as multi-targeted therapeutic agents, a series of synthetic indole-phenolic compounds was evaluated as multifunctional neuroprotectors. Each compound demonstrated metal-chelating properties, particularly in sequestering copper ions, with quantitative analysis revealing approximately 40% chelating activity across all the compounds. In cellular models, these hybrid compounds exhibited strong antioxidant and cytoprotective effects, countering reactive oxygen species (ROS) generated by the Aβ(25-35) peptide and its oxidative byproduct, hydrogen peroxide, as demonstrated by quantitative analysis showing on average a 25% increase in cell viability and a reduction in ROS levels to basal states. Further analysis using thioflavin T fluorescence assays, circular dichroism, and computational studies indicated that the synthesized derivatives effectively promoted the self-disaggregation of the Aβ(25-35) fragment. Taken together, these findings suggest a unique profile of neuroprotective actions for indole-phenolic derivatives, combining chelating, antioxidant, and anti-aggregation properties, which position them as promising compounds for the development of multifunctional agents in Alzheimer's disease therapy. The methods used provide reliable in vitro data, although further in vivo validation and assessment of blood-brain barrier penetration are needed to confirm therapeutic efficacy and safety.
Keywords: amyloid; antioxidants; disaggregation; in-cell studies; indole nucleus; neuroprotection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

