Circulating Tumor DNA for Prediction of Complete Pathological Response to Neoadjuvant Radiochemotherapy in Locally Advanced Rectal Cancer (NEORECT Trial)
- PMID: 39766073
- PMCID: PMC11674684
- DOI: 10.3390/cancers16244173
Circulating Tumor DNA for Prediction of Complete Pathological Response to Neoadjuvant Radiochemotherapy in Locally Advanced Rectal Cancer (NEORECT Trial)
Abstract
Background/objectives: Locally advanced rectal cancer is treated with neoadjuvant chemoradiotherapy (nCRT) followed by total mesorectal excision (TME). As this approach achieves complete pathologic remissions (pCR) in approximately 30% of patients, it raises the question of whether surgery is always necessary. Non-surgical strategies, such as "watch and wait" (W&W), have shown similarly promising outcomes. However, there is an unmet need for reliable biomarkers predicting pCR. Analysis of circulating tumor DNA (ctDNA) has shown potential for monitoring treatment response and detecting minimal residual disease. We hypothesized that monitoring ctDNA changes during nCRT might facilitate the identification of individuals who achieve pCR.
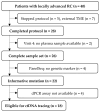
Methods: In the prospective single-center NEORECT trial, the plasma of forty rectal cancer patients was collected before, during, and after nCRT and before TME. Informative somatic mutations were identified in tissue biopsies by NGS and subsequently used for ctDNA quantification by dPCR.
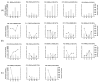
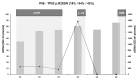
Results: The results identified three distinct ctDNA patterns: increase, decrease, and absence. Remarkably, undetectable DNA was observed in good responders, while a tenfold ctDNA increase was associated with the emergence of new metastases. Despite these insights, ctDNA alone demonstrated low specificity, with no significant correlation to pCR or long-term prognosis. A multimodal approach incorporating routinely available clinical parameters remains inadequate for accurately predicting pCR prior to TME.
Conclusions: In conclusion, the NEORECT trial establishes the feasibility of ctDNA-based personalized monitoring for rectal cancer patients undergoing nCRT. However, the utility of ctDNA in enhancing pCR prediction for a W&W strategy warrants further investigation. Larger studies integrating multi-gene analyses and expanded clinical datasets are essential in the future.
Keywords: circulating tumor DNA; complete pathological response; neoadjuvant radiochemotherapy; rectal cancer; response prediction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Garcia-Aguilar J., Patil S., Gollub M.J., Kim J.K., Yuval J.B., Thompson H.M., Verheij F.S., Omer D.M., Lee M., Dunne R.F., et al. Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy. J. Clin. Oncol. 2022;40:2546–2556. doi: 10.1200/JCO.22.00032. - DOI - PMC - PubMed
-
- Conroy T., Bosset J.F., Etienne P.L., Rio E., François É., Mesgouez-Nebout N., Vendrely V., Artignan X., Bouché O., Gargot D., et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:702–715. doi: 10.1016/S1470-2045(21)00079-6. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

