Chemokine CXCL12 Activates CXC Receptor 4 Metastasis Signaling Through the Upregulation of a CXCL12/CXCR4/MDMX (MDM4) Axis
- PMID: 39766093
- PMCID: PMC11674518
- DOI: 10.3390/cancers16244194
Chemokine CXCL12 Activates CXC Receptor 4 Metastasis Signaling Through the Upregulation of a CXCL12/CXCR4/MDMX (MDM4) Axis
Abstract
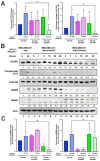
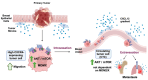
Background: The metastasis-promoting G-protein-coupled receptor CXC Receptor 4 (CXCR4) is activated by the chemokine CXCL12, also known as stromal cell-derived factor 1 (SDF-1). The CXCL12/CXCR4 pathway in cancer promotes metastasis but the molecular details of how this pathway cross-talks with oncogenes are understudied. An oncogene pathway known to promote breast cancer metastasis in MDA-MB-231 xenografts is that of Mouse Double Minute 2 and 4 (MDM2 and MDM4, also known as MDMX). MDM2 and MDMX promote circulating tumor cell (CTC) formation and metastasis, and positively correlate with a high expression of CXCR4. Interestingly, this MDMX-associated upregulation of CXCR4 is only observed in cells grown in the tumor microenvironment (TME), but not in MDA-MB-231 cells grown in a tissue culture dish. This suggested a cross-talk signaling factor from the TME which was predicted to be CXCL12 and, as such, we asked if the exogenous addition of the cell non-autonomous CXCL12 ligand would recapitulate the MDMX-dependent upregulation of CXCR4.
Methods: We used MDA-MB-231 cells and isolated CTCs, with and without MDMX knockdown, plus the exogenous addition of CXCL12 to determine if MDMX-dependent upregulation of CXCR4 could be recapitulated outside of the TME context. We added exogenous CXCL12 to the culture medium used for growth of MDA-MB-231 cells and isogenic cell lines engineered for MDM2 or MDMX depletion. We carried out immunoblotting, and quantitative RT-PCR to compare the expression of CXCR4, MDM2, MDMX, and AKT activation. We carried out Boyden chamber and wound healing assays to assess the influence of MDMX and CXCL12 on the cells' migration capacity.
Results: The addition of the CXCL12 chemokine to the medium increased the CXCR4 cellular protein level and activated the PI3K/AKT signaling pathway. Surprisingly, we observed that the addition of CXCL12 mediated the upregulation of MDM2 and MDMX at the protein, but not at the mRNA, level. A reduction in MDMX, but not MDM2, diminished both the CXCL12-mediated CXCR4 and MDM2 upregulation. Moreover, a reduction in both MDM2 and MDMX hindered the ability of the added CXCL12 to promote Boyden chamber-assessed cell migration. The upregulation of MDMX by CXCL12 was mediated, at least in part, by a step upstream of the proteasome pathway because CXCL12 did not increase protein stability after cycloheximide treatment, or when the proteasome pathway was blocked.
Conclusions: These data demonstrate a positive feed-forward activation loop between the CXCL12/CXCR4 pathway and the MDM2/MDMX pathway. As such, MDMX expression in tumor cells may be upregulated in the primary tumor microenvironment by CXCL12 expression. Furthermore, CXCL12/CXCR4 metastatic signaling may be upregulated by the MDM2/MDMX axis. Our findings highlight a novel positive regulatory loop between CXCL12/CXCR4 signaling and MDMX to promote metastasis.
Keywords: CXCR4; MDM2; MDMX; PI3K/AKT signaling; chemokine signaling; circulating tumor cells (CTCs); metastasis; triple-negative breast cancer (TNBC); tumor microenvironment (TME).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Xin Y., Hu B., Li K., Hu G., Zhang C., Chen X., Tang K., Du P., Tan Y. Circulating tumor cells with metastasis-initiating competence survive fluid shear stress during hematogenous dissemination through CXCR4-PI3K/AKT signaling. Cancer Lett. 2024;590:216870. doi: 10.1016/j.canlet.2024.216870. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

