Targeting Perineural Invasion in Pancreatic Cancer
- PMID: 39766161
- PMCID: PMC11674953
- DOI: 10.3390/cancers16244260
Targeting Perineural Invasion in Pancreatic Cancer
Abstract
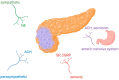
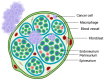
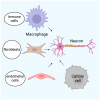
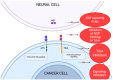
Pancreatic cancer is an aggressive tumor with dismal prognosis. Neural invasion is one of the pathological hallmarks of pancreatic cancer. Peripheral nerves can modulate the phenotype and behavior of the malignant cells, as well as of different components of the tumor microenvironment, and thus affect tumor growth and metastasis. From a clinical point of view, neural invasion is translated into intractable pain and represents a predictor of tumor recurrence and poor prognosis. Several molecules are implicated in neural invasion and pain onset in PDAC, including neutrophins (e.g., NGF), chemokines, adhesion factors, axon-guidance molecules, different proteins, and neurotransmitters. In this review, we discuss the role of nerves within the pancreatic cancer microenvironment, highlighting how infiltrating nerve fibers promote tumor progression and metastasis, while tumor cells, in turn, drive nerve outgrowth in a reciprocal interaction that fuels tumor advancement. We outline key molecules involved in neural invasion in pancreatic cancer and, finally, explore potential therapeutic strategies to target neural invasion, aiming to both inhibit cancer progression and alleviate cancer-associated pain.
Keywords: cancer pain; neural invasion; pancreatic ductal adenocarcinoma; tumor microenvironment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Faulkner S., Jobling P., March B., Jiang C.C., Hondermarck H. Tumor neurobiology and the war of nerves in cancer. Cancer Discov. 2019;9:702–710. doi: 10.1158/2159-8290.CD-18-1398. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

