Insights into the Relationship Between the Gut Microbiome and Immune Checkpoint Inhibitors in Solid Tumors
- PMID: 39766170
- PMCID: PMC11674129
- DOI: 10.3390/cancers16244271
Insights into the Relationship Between the Gut Microbiome and Immune Checkpoint Inhibitors in Solid Tumors
Abstract
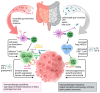
Immunotherapy with immune checkpoint inhibitors represents a revolutionary approach to the treatment of solid tumors, including malignant melanoma, lung cancer, and gastrointestinal malignancies. Anti-CTLA-4 and anti-PD-1/PDL-1 therapies provide prolonged survival for cancer patients, but their efficacy and safety are highly variable. This review focuses on the crucial role of the gut microbiome in modulating the efficacy and toxicity of immune checkpoint blockade. Studies suggest that the composition of the gut microbiome may influence the response to immunotherapy, with specific bacterial strains able to promote an anti-tumor immune response. On the other hand, dysbiosis may increase the risk of adverse effects, such as immune-mediated colitis. Interventions aimed at modulating the microbiome, including the use of probiotics, prebiotics, fecal microbial transplantation, or dietary modifications, represent promising strategies to increase treatment efficacy and reduce toxicity. The combination of immunotherapy with the microbiome-based strategy opens up new possibilities for personalized treatment. In addition, factors such as physical activity and nutritional supplementation may indirectly influence the gut ecosystem and consequently improve treatment outcomes in refractory patients, leading to enhanced patient responses and prolonged survival.
Keywords: cancer treatment; fecal microbiota transplantation; immune checkpoint inhibitors; probiotics; solid tumors; the gut microbiome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
Grants and funding
- 1/0071/24/Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and Slovak Academy of Sciences (VEGA)
- 2/0069/22/Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and Slovak Academy of Sciences (VEGA)
LinkOut - more resources
Full Text Sources

