Cardiotoxicity in Breast Cancer: Impact of Clinical Classifications and Treatment on Heart Health
- PMID: 39766180
- PMCID: PMC11674351
- DOI: 10.3390/cancers16244281
Cardiotoxicity in Breast Cancer: Impact of Clinical Classifications and Treatment on Heart Health
Abstract
Background/objectives: Cardio-oncology has become essential in addressing cardiovascular complications from cancer therapies. While advancements in treatments have improved survival rates, they also increase cardiovascular risks. This study evaluates the cardiotoxic effects of cytostatic treatments, examining the relationship between tumor characteristics, such as histopathology and TNM classification, and cardiovascular complications, aiming to improve cardiotoxicity prevention and management in oncology patients.
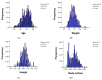
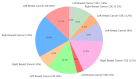
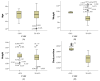
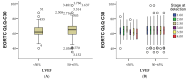
Methods: We conducted a retrospective analysis of cancer patients treated with anthracyclines, HER2-targeted therapies, and radiotherapy. Cardiac function was monitored via echocardiography, focusing on global longitudinal strain and left ventricular ejection fraction (LVEF). Cardiac troponins and natriuretic peptides were measured to detect subclinical cardiotoxicity, with patients stratified by TNM cancer stage and histopathology.
Results: Our analysis identified a significant association between certain cytostatic treatments, such as anthracyclines and HER2-targeted therapies, and a reduction in LVEF, particularly in patients with advanced-stage cancer. Biomarker assessments indicated early signs of cardiotoxicity in patients before clinical symptoms emerged. The findings also demonstrated a higher prevalence of cardiovascular complications in patients with pre-existing risk factors.
Conclusions: This study highlights the importance of personalized treatment protocols in minimizing cardiotoxicity and improving the quality of life for oncology patients. Regular cardiac monitoring, combined with the use of biomarkers, can help identify high-risk patients early, allowing for timely interventions. Future research should focus on optimizing cardioprotective strategies to mitigate the cardiovascular risks associated with modern cancer therapies.
Clinical trial registration: N/A (retrospective study).
Keywords: HER2-targeted therapies; cancer therapies; cardiotoxicity; chemotherapy-induced heart failure; left ventricular ejection fraction (LVEF).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Association Between Advanced TNM Stages and Increased Risk of Cardiac Dysfunction in Patients with LVEF < 50.Medicina (Kaunas). 2025 Feb 10;61(2):301. doi: 10.3390/medicina61020301. Medicina (Kaunas). 2025. PMID: 40005419 Free PMC article.
-
Trastuzumab-induced cardiotoxicity: a review of clinical risk factors, pharmacologic prevention, and cardiotoxicity of other HER2-directed therapies.Breast Cancer Res Treat. 2021 Jul;188(1):21-36. doi: 10.1007/s10549-021-06280-x. Epub 2021 Jun 11. Breast Cancer Res Treat. 2021. PMID: 34115243 Review.
-
A Systematic Review of the Cardiotoxic Effects of Targeted Therapies in Oncology.Cureus. 2024 Aug 6;16(8):e66258. doi: 10.7759/cureus.66258. eCollection 2024 Aug. Cureus. 2024. PMID: 39238728 Free PMC article. Review.
-
A randomized trial to evaluate the impact of exercise-based cardiac rehabilitation for the prevention of chemotherapy-induced cardiotoxicity in patients with breast cancer: ONCORE study protocol.BMC Cardiovasc Disord. 2021 Apr 7;21(1):165. doi: 10.1186/s12872-021-01970-2. BMC Cardiovasc Disord. 2021. PMID: 33827450 Free PMC article.
-
Cardiotoxic Effects of HER2-Targeted Therapies: Insights From a Retrospective Study at a Romanian Oncology Center.Cureus. 2025 Apr 25;17(4):e83012. doi: 10.7759/cureus.83012. eCollection 2025 Apr. Cureus. 2025. PMID: 40421343 Free PMC article.
Cited by
-
Development and validation of a predictive model for cancer therapy-related cardiac dysfunction in breast cancer patients using echocardiographic indicators.Am J Cancer Res. 2025 May 15;15(5):2243-2258. doi: 10.62347/WPUW2205. eCollection 2025. Am J Cancer Res. 2025. PMID: 40520878 Free PMC article.
-
F-α-DDB-derivative, a novel synthetic of bifendate, plus epirubicin improves antitumor efficacy against triple negative breast cancer without additional cardiotoxicity.Discov Oncol. 2025 May 8;16(1):690. doi: 10.1007/s12672-025-02545-9. Discov Oncol. 2025. PMID: 40338489 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

