Diabetic Glycation of Human Serum Albumin Affects Its Immunogenicity
- PMID: 39766199
- PMCID: PMC11673269
- DOI: 10.3390/biom14121492
Diabetic Glycation of Human Serum Albumin Affects Its Immunogenicity
Abstract
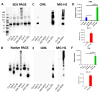
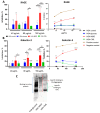
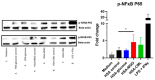
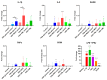
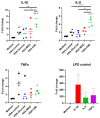
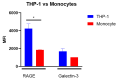
Advanced glycation end-products (AGEs) are products of a non-enzymatic reaction between amino acids and reducing sugars. Glycated human serum albumin (HSA) increases in diabetics as a consequence of elevated blood glucose levels and glycating metabolites like methylglyoxal (MGO). The impact of different types of glycation on the immunomodulatory properties of HSA is poorly understood and is studied here. HSA was glycated with D-glucose, MGO, or glyoxylic acid (CML). Glycation-related biochemical changes were characterized using various biochemical methods. The binding of differentially glycated HSA to AGE receptors was determined with inhibition ELISAs, and the impact on inflammatory markers in macrophage cell line THP-1 and adherent monocytes isolated from human peripheral blood mononuclear cells (PBMCs) was studied. All glycation methods led to unique AGE profiles and had a distinct impact on protein structure. Glycation resulted in increased binding of HSA to the AGE receptors, with MGO modification showing the highest binding, followed by glucose and, lastly, CML. Additionally, modification of HSA with MGO led to the increased expression of pro-inflammatory markers in THP-1 macrophages and enhanced phosphorylation of NF-κB p65. The same pattern, although less prominent, was observed for HSA glycated with glucose and CML, respectively. An increase in pro-inflammatory markers was also observed in PBMC-derived monocytes exposed to all glycated forms of HSA, although HSA-CML led to a significantly higher inflammatory response. In conclusion, the type of HSA glycation impacts immune functional readouts with potential relevance for diabetes.
Keywords: AGEs; HSA; RAGE; diabetes; inflammation; macrophages; methylglyoxal; receptors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Kumari N., Bandyopadhyay D., Kumar V., Venkatesh D.B., Prasad S., Prakash S., Krishnaswamy P.R., Balaram P., Bhat N. Glycation of albumin and its implication in Diabetes: A comprehensive analysis using mass spectrometry. Clin. Chim. Acta. 2021;520:108–117. doi: 10.1016/j.cca.2021.06.001. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

