Unlocking Testosterone Production by Biotransformation: Engineering a Fungal Model of Aspergillus nidulans Strain Deficient in Steroid 11α-Hydroxylase Activity and Expressing 17β-Hydroxysteroid Dehydrogenase Enzyme as Proof of Concept
- PMID: 39766209
- PMCID: PMC11673057
- DOI: 10.3390/biom14121502
Unlocking Testosterone Production by Biotransformation: Engineering a Fungal Model of Aspergillus nidulans Strain Deficient in Steroid 11α-Hydroxylase Activity and Expressing 17β-Hydroxysteroid Dehydrogenase Enzyme as Proof of Concept
Abstract
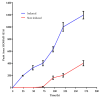
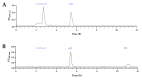
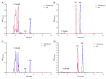
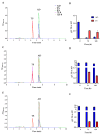
Testosterone holds significant medical and economic importance, with the global market for testosterone replacement therapies valued at approximately USD 1.9 billion in 2023. This hormone is essential for the development and maintenance of male sexual characteristics as well as bone and muscle health. It plays a key role in conditions such as hypogonadism, muscle disorders, and andropause. However, the industrial production of testosterone often involves complex chemical processes that result in low yields, high costs, and environmental damage. Microbial biotransformation of steroids presents an eco-friendly alternative to traditional chemical synthesis. A knockout strain of Aspergillus nidulans deficient in steroid 11α-hydroxylase activity was developed, rendering it incapable of hydroxylating androstenedione, progesterone, and testosterone. In these strains, two newly identified CYP450 enzymes, CYP68L1 from A. nidulans and CYP68L8 from Aspergillus ochraceus, were expressed to confirm their roles as steroid 11α-hydroxylases of androstenedione, progesterone, and testosterone. The availability of these 11α-hydroxylases represents significant progress toward achieving efficient single-step steroid fermentation. Furthermore, the A. nidulans knockout strain serves as an effective model for studying the conversion of androstenedione to testosterone upon the expression of the enzyme 17β-hydroxysteroid dehydrogenase, due to its inability to hydroxylate testosterone.
Keywords: 17β-hydroxysteroid dehydrogenase (17β-HSD) EC 1.1.1.51; Aspergillus nidulans; Aspergillus ochraceus; androstenedione; progesterone; steroid 11α-hydroxylase; testosterone.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Biotransformation of progesterone by Aspergillus nidulans VKPM F-1069 (wild type).Steroids. 2019 Sep;149:108421. doi: 10.1016/j.steroids.2019.05.013. Epub 2019 Jun 6. Steroids. 2019. PMID: 31176657
-
The lithocholic acid 6 beta-hydroxylase cytochrome P-450, CYP 3A10, is an active catalyst of steroid-hormone 6 beta-hydroxylation.Biochem J. 1993 Apr 15;291 ( Pt 2)(Pt 2):429-33. doi: 10.1042/bj2910429. Biochem J. 1993. PMID: 8484723 Free PMC article.
-
Expression of steroid-converting enzymes in osteoblasts derived from rat vertebrae.Osteoporos Int. 2002 Mar;13(3):235-40. doi: 10.1007/s001980200020. Osteoporos Int. 2002. PMID: 11991444
-
Steroid-transforming enzymes in fungi.J Steroid Biochem Mol Biol. 2012 Mar;129(1-2):79-91. doi: 10.1016/j.jsbmb.2011.08.012. Epub 2011 Sep 14. J Steroid Biochem Mol Biol. 2012. PMID: 21946531 Review.
-
Steroid 17β-hydroxysteroid dehydrogenase deficiency in man: an inherited form of male pseudohermaphroditism.J Steroid Biochem Mol Biol. 1992 Dec;43(8):989-1002. doi: 10.1016/0960-0760(92)90327-F. J Steroid Biochem Mol Biol. 1992. PMID: 22217844 Review.
Cited by
-
Association between the oxidative balance score and testosterone deficiency in males: a cross-sectional study.Front Nutr. 2025 Jul 23;12:1577823. doi: 10.3389/fnut.2025.1577823. eCollection 2025. Front Nutr. 2025. PMID: 40771217 Free PMC article.
References
-
- Kitamura H., Takahashi A., Hotta H., Kato R., Kunishima Y., Takei F., Horita H., Masumori N., Oncology S.M.U.U. Palonosetron with aprepitant plus dexamethasone to prevent chemotherapy-induced nausea and vomiting during gemcitabine/cisplatin in urothelial cancer patients. Int. J. Urol. 2015;22:911–914. doi: 10.1111/iju.12842. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

