Steroids and Malignancy Increase Local Heparanase and Decrease Markers of Osteoblast Activity in Bone Tissue Microcirculation
- PMID: 39766213
- PMCID: PMC11673960
- DOI: 10.3390/biom14121506
Steroids and Malignancy Increase Local Heparanase and Decrease Markers of Osteoblast Activity in Bone Tissue Microcirculation
Abstract
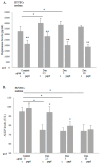
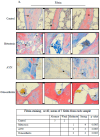
Bone metastasis and steroids are known to activate the coagulation system and induce osteoporosis, pathological bone fractures, and bone pain. Heparanase is a protein known to enhance the hemostatic system and to promote angiogenesis, metastasis, and inflammation. The objective of the present study was to evaluate the effects of steroids and malignancy on the coagulation factors and osteoblast activity in the bone tissue. The effects of dexacort and malignant medium were evaluated in osteoblasts derived from human bone marrow mesenchymal stem cells and human umbilical vein endothelial cells (HUVECs). The bones of mice treated with dexacort for 1 month were studied. Bone biopsies of ten patients with bone metastasis, ten with steroid-induced avascular necrosis (AVN), and ten with osteoarthritis were compared to ten controls. We found that dexacort and malignant medium significantly increased the heparanase levels in osteoblasts and HUVECs and decreased the levels of alkaline phosphatase (ALKP). Peptide 16AC, derived from heparanase, which interacts with tissue factor (TF), further increased the effect, while peptide 6, which inhibits interactions between heparanase and TF, reversed the effect in these cells. The bone microcirculation of mice treated with dexacort exhibited significantly higher levels of heparanase, TF, TF pathway inhibitor (TFPI), TFPI-2, thrombin, and syndecan-1, but reduced levels of osteocalcin and ALKP. The pathological human bone biopsies' microcirculation exhibited significantly dilated blood vessels and higher levels of heparanase, TF, TFPI, TFPI-2, and fibrin. In summary, steroids and malignancy increased the activation of the coagulation system in the bone microcirculation and reduced the osteoblast activity. Heparanase inhibitors should be further investigated to attenuate bone fractures and pain.
Keywords: bone; heparanase; malignancy; microcirculation; steroids; thrombosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures











Similar articles
-
Effect of bone marrow blood versus peripheral blood on the hemostatic balance of osteoblasts and endothelial cells.Sci Rep. 2025 Apr 21;15(1):13713. doi: 10.1038/s41598-025-94942-x. Sci Rep. 2025. PMID: 40258877 Free PMC article.
-
Heparanase Level in the Microcirculation as a Possible Modulator of the Metastatic Process.Am J Pathol. 2019 Aug;189(8):1654-1663. doi: 10.1016/j.ajpath.2019.04.019. Epub 2019 May 23. Am J Pathol. 2019. PMID: 31128083
-
Heparanase induces tissue factor pathway inhibitor expression and extracellular accumulation in endothelial and tumor cells.Thromb Haemost. 2008 Jan;99(1):133-41. Thromb Haemost. 2008. PMID: 18217145
-
Heparanase in the Coagulation System.Adv Exp Med Biol. 2020;1221:771-784. doi: 10.1007/978-3-030-34521-1_33. Adv Exp Med Biol. 2020. PMID: 32274737 Review.
-
Heparanase coagulation and cancer progression.Best Pract Res Clin Haematol. 2009 Mar;22(1):85-92. doi: 10.1016/j.beha.2008.12.004. Best Pract Res Clin Haematol. 2009. PMID: 19285275 Review.
Cited by
-
Mediating Role of the ANGPTL3/TFPI Protein Ratio in Regulating T-Cell Surface Glycoprotein CD5 Levels on Knee Osteoarthritis (KOA): A Mendelian Randomization Study.Int J Mol Sci. 2025 May 8;26(10):4471. doi: 10.3390/ijms26104471. Int J Mol Sci. 2025. PMID: 40429617 Free PMC article.
References
-
- Feingold K.R., Anawalt B., Blackman M.R., Boyce A., Chrousos G., Corpas E., de Herder W.W., Dhatariya K., Dungan K., Hofland J., et al., editors. Endotext. MDText.com, Inc.; South Dartmouth, MA, USA: 2000. An Overview of Glucocorticoid-Induced Osteoporosis. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

