Senescence-like Phenotype After Chronic Exposure to Isoproterenol in Primary Quiescent Immune Cells
- PMID: 39766235
- PMCID: PMC11673961
- DOI: 10.3390/biom14121528
Senescence-like Phenotype After Chronic Exposure to Isoproterenol in Primary Quiescent Immune Cells
Abstract
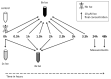
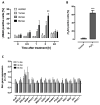
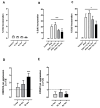
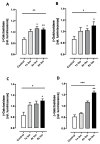
Chronic stress is associated with a higher risk for carcinogenesis as well as age-related diseases and immune dysfunction. There is evidence showing that psychological stress can contribute to premature immunosenescence. Therefore, the question arose whether chronic exposure to catecholamine could drive immune cells into senescence. Peripheral blood mononuclear cells were isolated from whole blood. After repeated ex vivo treatment with isoproterenol, an epinephrine analog, well-established senescence biomarkers were assessed. We found (i) DNA double-strand break induction, (ii) telomere shortening, (iii) failure to proliferate, (iv) higher senescence-associated β-galactosidase activity, (v) decreases in caspases 3 and 7 activity, and (vi) strong upregulation of the proteoglycan versican accompanied by increased cellular adhesion suggesting the induction of a senescence-like phenotype. These results emphasize the complexity of the effect of isoproterenol on multiple cellular processes and provide insights into the molecular mechanisms of stress leading to immunosenescence.
Keywords: DNA strand breaks; catecholamine; cellular senescence; isoproterenol; β-galactosidase activity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Antonini S.R., Stecchini M.F., Ramalho F.S. Development and Function of the Adrenal Cortex and Medulla in the Fetus and Neonate. In: Deal C.S.K.a.C.L., editor. Maternal-Fetal and Neonatal Endocrinology Physiology, Pathophysiology, and Clinical Management. Elsevier; Amsterdam, The Netherlands: 2020. pp. 611–623.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

