Higher-Order DNA Secondary Structures and Their Transformations: The Hidden Complexities of Tetrad and Quadruplex DNA Structures, Complexes, and Modulatory Interactions Induced by Strand Invasion Events
- PMID: 39766239
- PMCID: PMC11673204
- DOI: 10.3390/biom14121532
Higher-Order DNA Secondary Structures and Their Transformations: The Hidden Complexities of Tetrad and Quadruplex DNA Structures, Complexes, and Modulatory Interactions Induced by Strand Invasion Events
Abstract
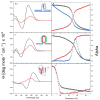
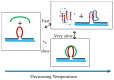
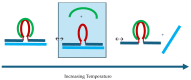
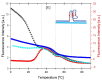
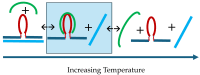
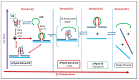
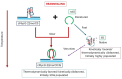
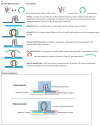
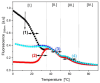
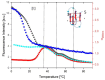
We demonstrate that a short oligonucleotide complementary to a G-quadruplex domain can invade this iconic, noncanonical DNA secondary structure in ways that profoundly influence the properties and differential occupancies of the resulting DNA polymorphic products. Our spectroscopic mapping of the conformational space of the associated reactants and products, both before and after strand invasion, yield unanticipated outcomes which reveal several overarching features. First, strand invasion induces the disruption of DNA secondary structural elements in both the invading strand (which can assume an iDNA tetrad structure) and the invaded species (a G-quadruplex). The resultant cascade of coupled alterations represents a potential pathway for the controlled unfolding of kinetically trapped DNA states, a feature that may be characteristic of biological regulatory mechanisms. Furthermore, the addition of selectively designed, exogenous invading oligonucleotides can enable the manipulation of noncanonical DNA conformations for biomedical applications. Secondly, our results highlight the importance of metastability, including the interplay between slower and faster kinetic processes in determining preferentially populated DNA states. Collectively, our data reveal the importance of sample history in defining state populations, which, in turn, determine preferred pathways for further folding steps, irrespective of the position of the thermodynamic equilibrium. Finally, our spectroscopic data reveal the impact of topological constraints on the differential stabilities of base-paired domains. We discuss how our collective observations yield insights into the coupled and uncoupled cascade of strand-invasion-induced transformations between noncanonical DNA forms, potentially as components of molecular wiring diagrams that regulate biological processes.
Keywords: DNA self-regulation pathways; DNA strand invasion; G-quadruplex/iDNA/duplex interconversions; competing kinetic and thermodynamic states; coupled transformations of higher-order DNA states; metastable DNA states; rough energy landscapes.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures













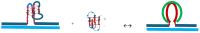




References
-
- Wales D.J. Energy Landscapes Applications to Clusters, Biomolecules and Glasses. University of Cambridge; Cambridge, UK: 2004.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

