Mitochondrial Dysfunction in Cardiac Disease: The Fort Fell
- PMID: 39766241
- PMCID: PMC11673776
- DOI: 10.3390/biom14121534
Mitochondrial Dysfunction in Cardiac Disease: The Fort Fell
Abstract
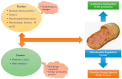
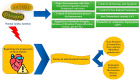
Myocardial cells and the extracellular matrix achieve their functions through the availability of energy. In fact, the mechanical and electrical properties of the heart are heavily dependent on the balance between energy production and consumption. The energy produced is utilized in various forms, including kinetic, dynamic, and thermal energy. Although total energy remains nearly constant, the contribution of each form changes over time. Thermal energy increases, while dynamic and kinetic energy decrease, ultimately becoming insufficient to adequately support cardiac function. As a result, toxic byproducts, unfolded or misfolded proteins, free radicals, and other harmful substances accumulate within the myocardium. This leads to the failure of crucial processes such as myocardial contraction-relaxation coupling, ion exchange, cell growth, and regulation of apoptosis and necrosis. Consequently, both the micro- and macro-architecture of the heart are altered. Energy production and consumption depend on the heart's metabolic resources and the functional state of the cardiac structure, including cardiomyocytes, non-cardiomyocyte cells, and their metabolic and energetic behavior. Mitochondria, which are intracellular organelles that produce more than 95% of ATP, play a critical role in fulfilling all these requirements. Therefore, it is essential to gain a deeper understanding of their anatomy, function, and homeostatic properties.
Keywords: cardiac disease; energy; heart failure; mitochondria.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Energetic Dysfunction Is Mediated by Mitochondrial Reactive Oxygen Species and Precedes Structural Remodeling in Metabolic Heart Disease.Antioxid Redox Signal. 2019 Sep 1;31(7):539-549. doi: 10.1089/ars.2018.7707. Epub 2019 Jun 25. Antioxid Redox Signal. 2019. PMID: 31088291 Free PMC article.
-
Mitochondrial transfer in the progression and treatment of cardiac disease.Life Sci. 2024 Dec 1;358:123119. doi: 10.1016/j.lfs.2024.123119. Epub 2024 Oct 10. Life Sci. 2024. PMID: 39395616 Review.
-
Mitochondria in Structural and Functional Cardiac Remodeling.Adv Exp Med Biol. 2017;982:277-306. doi: 10.1007/978-3-319-55330-6_15. Adv Exp Med Biol. 2017. PMID: 28551793 Review.
-
Mitochondrial oxidative metabolism and uncoupling proteins in the failing heart.Heart Fail Rev. 2015 Mar;20(2):227-49. doi: 10.1007/s10741-014-9457-4. Heart Fail Rev. 2015. PMID: 25192828 Review.
-
Mitochondrial remodeling in mice with cardiomyocyte-specific lipid overload.J Mol Cell Cardiol. 2015 Feb;79:275-83. doi: 10.1016/j.yjmcc.2014.12.001. Epub 2014 Dec 9. J Mol Cell Cardiol. 2015. PMID: 25497302 Free PMC article.
Cited by
-
Stellate Ganglia: A Key Therapeutic Target for Malignant Ventricular Arrhythmia in Heart Disease.Circ Res. 2025 Apr 25;136(9):1049-1069. doi: 10.1161/CIRCRESAHA.124.325384. Epub 2025 Apr 24. Circ Res. 2025. PMID: 40273204 Review.
-
Hypertrophic Cardiomyopathy Through the Lens of Mitochondria.Biomedicines. 2025 Feb 28;13(3):591. doi: 10.3390/biomedicines13030591. Biomedicines. 2025. PMID: 40149568 Free PMC article. Review.
-
Exosome: an overview on enhanced biogenesis by small molecules.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jun;398(6):6473-6508. doi: 10.1007/s00210-024-03762-9. Epub 2025 Jan 25. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39862264 Review.
-
Molecular and Biochemical Mechanisms of Cardiomyopathy Development Following Prenatal Hypoxia-Focus on the NO System.Antioxidants (Basel). 2025 Jun 16;14(6):743. doi: 10.3390/antiox14060743. Antioxidants (Basel). 2025. PMID: 40563375 Free PMC article. Review.
References
-
- Neubauer S. The Failing Heart—An Engine Out of Fuel. N. Engl. J. Med. 2007;356:1140–1151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

