Role of the Receptor for Advanced Glycation End Products (RAGE) and Its Ligands in Inflammatory Responses
- PMID: 39766257
- PMCID: PMC11673996
- DOI: 10.3390/biom14121550
Role of the Receptor for Advanced Glycation End Products (RAGE) and Its Ligands in Inflammatory Responses
Abstract
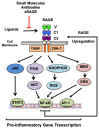
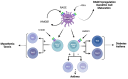
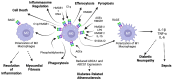
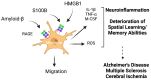
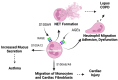
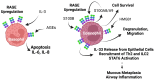
Since its discovery in 1992, the receptor for advanced glycation end products (RAGE) has emerged as a key receptor in many pathological conditions, especially in inflammatory conditions. RAGE is expressed by most, if not all, immune cells and can be activated by many ligands. One characteristic of RAGE is that its ligands are structurally very diverse and belong to different classes of molecules, making RAGE a promiscuous receptor. Many of RAGE ligands are damaged associated molecular patterns (DAMPs) that are released by cells under inflammatory conditions. Although RAGE has been at the center of a lot of research in the past three decades, a clear understanding of the mechanisms of RAGE activation by its ligands is still missing. In this review, we summarize the current knowledge of the role of RAGE and its ligands in inflammation.
Keywords: DAMP; RAGE; S100; inflammation; stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Glycation reaction and the role of the receptor for advanced glycation end-products in immunity and social behavior.Glycoconj J. 2021 Jun;38(3):303-310. doi: 10.1007/s10719-020-09956-6. Epub 2020 Oct 27. Glycoconj J. 2021. PMID: 33108607 Review.
-
Targeting RAGE Signaling in Inflammatory Disease.Annu Rev Med. 2018 Jan 29;69:349-364. doi: 10.1146/annurev-med-041316-085215. Epub 2017 Nov 6. Annu Rev Med. 2018. PMID: 29106804 Review.
-
The receptor for advanced glycation end-products (RAGE) is an important pattern recognition receptor (PRR) for inflammaging.Biogerontology. 2019 Jun;20(3):279-301. doi: 10.1007/s10522-019-09808-3. Epub 2019 Apr 9. Biogerontology. 2019. PMID: 30968282 Review.
-
RAGE and its ligands: from pathogenesis to therapeutics.Crit Rev Biochem Mol Biol. 2020 Dec;55(6):555-575. doi: 10.1080/10409238.2020.1819194. Epub 2020 Sep 16. Crit Rev Biochem Mol Biol. 2020. PMID: 32933340
-
Activation of the receptor for advanced glycation end products and consequences on health.Diabetes Metab Syndr. 2017 Oct-Dec;11(4):305-309. doi: 10.1016/j.dsx.2016.09.009. Epub 2016 Sep 4. Diabetes Metab Syndr. 2017. PMID: 27612394 Review.
Cited by
-
Effects of Exercise on Dynamic Balance in People with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.Life (Basel). 2025 Jun 4;15(6):913. doi: 10.3390/life15060913. Life (Basel). 2025. PMID: 40566565 Free PMC article. Review.
-
Randomized, double-blind, placebo-controlled pilot study of metformin as an adjunctive therapy in Parkinson's disease.Front Pharmacol. 2025 May 2;16:1497261. doi: 10.3389/fphar.2025.1497261. eCollection 2025. Front Pharmacol. 2025. PMID: 40385486 Free PMC article.
References
-
- Fehrenbach H., Kasper M., Tschernig T., Shearman M.S., Schuh D., Muller M. Receptor for Advanced Glycation Endproducts (RAGE) Exhibits Highly Differential Cellular and Subcellular Localisation in Rat and Human Lung. Cell. Mol. Biol. 1998;44:1147–1157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

