Organ-on-a-Chip Models-New Possibilities in Experimental Science and Disease Modeling
- PMID: 39766276
- PMCID: PMC11674024
- DOI: 10.3390/biom14121569
Organ-on-a-Chip Models-New Possibilities in Experimental Science and Disease Modeling
Abstract
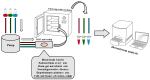
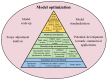
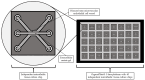
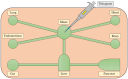
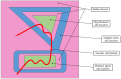
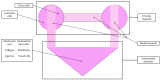
'Organ-on-a-chip' technology is a promising and rapidly evolving model in biological research. This innovative microfluidic cell culture device was created using a microchip with continuously perfused chambers, populated by living cells arranged to replicate physiological processes at the tissue and organ levels. By consolidating multicellular structures, tissue-tissue interfaces, and physicochemical microenvironments, these microchips can replicate key organ functions. They also enable the high-resolution, real-time imaging and analysis of the biochemical, genetic, and metabolic activities of living cells in the functional tissue and organ contexts. This technology can accelerate research into tissue development, organ physiology and disease etiology, therapeutic approaches, and drug testing. It enables the replication of entire organ functions (e.g., liver-on-a-chip, hypothalamus-pituitary-on-a-chip) or the creation of disease models (e.g., amyotrophic lateral sclerosis-on-a-chip, Parkinson's disease-on-a-chip) using specialized microchips and combining them into an integrated functional system. This technology allows for a significant reduction in the number of animals used in experiments, high reproducibility of results, and the possibility of simultaneous use of multiple cell types in a single model. However, its application requires specialized equipment, advanced expertise, and currently incurs high costs. Additionally, achieving the level of standardization needed for commercialization remains a challenge at this stage of development.
Keywords: microfluidics; microphysiological system; neurodegenerative diseases; neuroendocrinology; organ-on-a-chip.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

