Endoplasmic Reticulum Calcium Signaling in Hippocampal Neurons
- PMID: 39766324
- PMCID: PMC11727531
- DOI: 10.3390/biom14121617
Endoplasmic Reticulum Calcium Signaling in Hippocampal Neurons
Abstract
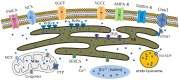
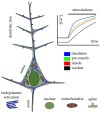
The endoplasmic reticulum (ER) is a key organelle in cellular homeostasis, regulating calcium levels and coordinating protein synthesis and folding. In neurons, the ER forms interconnected sheets and tubules that facilitate the propagation of calcium-based signals. Calcium plays a central role in the modulation and regulation of numerous functions in excitable cells. It is a versatile signaling molecule that influences neurotransmitter release, muscle contraction, gene expression, and cell survival. This review focuses on the intricate dynamics of calcium signaling in hippocampal neurons, with particular emphasis on the activation of voltage-gated and ionotropic glutamate receptors in the plasma membrane and ryanodine and inositol 1,4,5-trisphosphate receptors in the ER. These channels and receptors are involved in the generation and transmission of electrical signals and the modulation of calcium concentrations within the neuronal network. By analyzing calcium fluctuations in neurons and the associated calcium handling mechanisms at the ER, mitochondria, endo-lysosome and cytosol, we can gain a deeper understanding of the mechanistic pathways underlying neuronal interactions and information transfer.
Keywords: calcium signaling; endoplasmic reticulum; inositol 1,4,5-trisphosphate receptor; neurons; ryanodine receptor.
Conflict of interest statement
The author declares no conflicts of interest.
Figures
Similar articles
-
Neuregulin beta1 enhances peak glutamate-induced intracellular calcium levels through endoplasmic reticulum calcium release in cultured hippocampal neurons.Can J Physiol Pharmacol. 2009 Oct;87(10):883-91. doi: 10.1139/Y09-082. Can J Physiol Pharmacol. 2009. PMID: 20052014
-
Ryanodine receptor-mediated Ca2+ release and atlastin-2 GTPase activity contribute to IP3-induced dendritic Ca2+ signals in primary hippocampal neurons.Cell Calcium. 2021 Jun;96:102399. doi: 10.1016/j.ceca.2021.102399. Epub 2021 Mar 23. Cell Calcium. 2021. PMID: 33812310
-
Disruption of endoplasmic reticulum calcium stores is involved in neuronal death induced by glycolysis inhibition in cultured hippocampal neurons.J Neurosci Res. 2005 Oct 15;82(2):196-205. doi: 10.1002/jnr.20631. J Neurosci Res. 2005. PMID: 16175570
-
Contribution of Ca2+ release channels to hippocampal synaptic plasticity and spatial memory: potential redox modulation.Antioxid Redox Signal. 2014 Aug 20;21(6):892-914. doi: 10.1089/ars.2013.5796. Epub 2014 Mar 11. Antioxid Redox Signal. 2014. PMID: 24410659 Review.
-
Neuronal modeling with intracellular calcium signaling.Sheng Li Xue Bao. 2011 Oct 25;63(5):442-52. Sheng Li Xue Bao. 2011. PMID: 22002235 Review.
Cited by
-
Muscarinic Receptor Antagonism and TRPM3 Activation as Stimulators of Mitochondrial Function and Axonal Repair in Diabetic Sensorimotor Polyneuropathy.Int J Mol Sci. 2025 Jul 31;26(15):7393. doi: 10.3390/ijms26157393. Int J Mol Sci. 2025. PMID: 40806522 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

