Gramicidin A in Asymmetric Lipid Membranes
- PMID: 39766349
- PMCID: PMC11727149
- DOI: 10.3390/biom14121642
Gramicidin A in Asymmetric Lipid Membranes
Abstract
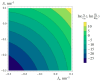
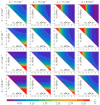
Gramicidin A is a natural antimicrobial peptide produced by Bacillus brevis. Its transmembrane dimer is a cation-selective ion channel. The channel is characterized by the average lifetime of the conducting state and the monomer-dimer equilibrium constant. Dimer formation is accompanied by deformations of the membrane. We theoretically studied how the asymmetry in lipid membrane monolayers influences the formation of the gramicidin A channel. We calculated how the asymmetry in the spontaneous curvature and/or lateral tension of lipid monolayers changes the channel lifetime and shifts the equilibrium constant of the dimerization/dissociation process. For the asymmetry expected to arise in plasma membranes of mammalian cells upon the addition of gramicidin A or its derivatives to the cell exterior, our model predicts a manifold increase in the average lifetime and equilibrium constant.
Keywords: asymmetric lipid membrane; channel lifetime; equilibrium constant; gramicidin A; intrinsic curvature; lateral tension; lipid–protein interaction; membrane biophysics; theory of elasticity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

