Alpha-Synuclein Effects on Mitochondrial Quality Control in Parkinson's Disease
- PMID: 39766356
- PMCID: PMC11674454
- DOI: 10.3390/biom14121649
Alpha-Synuclein Effects on Mitochondrial Quality Control in Parkinson's Disease
Abstract
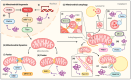
The maintenance of healthy mitochondria is essential for neuronal survival and relies upon mitochondrial quality control pathways involved in mitochondrial biogenesis, mitochondrial dynamics, and mitochondrial autophagy (mitophagy). Mitochondrial dysfunction is critically implicated in Parkinson's disease (PD), a brain disorder characterized by the progressive loss of dopaminergic neurons in the substantia nigra. Consequently, impaired mitochondrial quality control may play a key role in PD pathology. This is affirmed by work indicating that genes such as PRKN and PINK1, which participate in multiple mitochondrial processes, harbor PD-associated mutations. Furthermore, mitochondrial complex-I-inhibiting toxins like MPTP and rotenone are known to cause Parkinson-like symptoms. At the heart of PD is alpha-synuclein (αS), a small synaptic protein that misfolds and aggregates to form the disease's hallmark Lewy bodies. The specific mechanisms through which aggregated αS exerts its neurotoxicity are still unknown; however, given the vital role of both αS and mitochondria to PD, an understanding of how αS influences mitochondrial maintenance may be essential to elucidating PD pathogenesis and discovering future therapeutic targets. Here, the current knowledge of the relationship between αS and mitochondrial quality control pathways in PD is reviewed, highlighting recent findings regarding αS effects on mitochondrial biogenesis, dynamics, and autophagy.
Keywords: PGC-1α; PINK1/Parkin; Parkinson’s disease; mitochondrial dysfunction; mitochondrial fragmentation; mitophagy; α-synuclein.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Dorsey E.R., Elbaz A., Nichols E., Abbasi N., Abd-Allah F., Abdelalim A., Adsuar J.C., Ansha M.G., Brayne C., Choi J.-Y.J., et al. Global, Regional, and National Burden of Parkinson’s Disease, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–953. doi: 10.1016/S1474-4422(18)30295-3. - DOI - PMC - PubMed
-
- Kouli A., Torsney K.M., Kuan W.-L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In: Stoker T.B., Greenland J.C., editors. Parkinson’s Disease: Pathogenesis and Clinical Aspects. Codon Publications; Brisbane, Australia: 2018. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

