Gut Microbiota Modulation: A Novel Strategy for Rheumatoid Arthritis Therapy
- PMID: 39766360
- PMCID: PMC11674688
- DOI: 10.3390/biom14121653
Gut Microbiota Modulation: A Novel Strategy for Rheumatoid Arthritis Therapy
Abstract
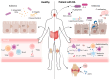
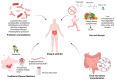
Rheumatoid arthritis (RA) is a chronic autoimmune disease that leads to joint inflammation, progressive tissue damage and significant disability, severely impacting patients' quality of life. While the exact mechanisms underlying RA remain elusive, growing evidence suggests a strong link between intestinal microbiota dysbiosis and the disease's development and progression. Differences in microbial composition between healthy individuals and RA patients point to the role of gut microbiota in modulating immune responses and promoting inflammation. Therapies targeting microbiota restoration have demonstrated promise in improving treatment efficacy, enhancing patient outcomes and slowing disease progression. However, the complex interplay between gut microbiota and autoimmune pathways in RA requires further investigation to establish causative relationships and mechanisms. Here, we review the current understanding of the gut microbiota's role in RA pathogenesis and its potential as a therapeutic target.
Keywords: bacterial metabolites; bacterial translocation; dysbiosis; gut microbiota; molecular mimicry; rheumatoid arthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Microbial imbalance in the gut: a new frontier in Rheumatoid arthritis research.Inflammopharmacology. 2025 May;33(5):2277-2291. doi: 10.1007/s10787-025-01737-7. Epub 2025 Apr 12. Inflammopharmacology. 2025. PMID: 40220199 Review.
-
Microbiota and immune dynamics in rheumatoid arthritis: Mechanisms and therapeutic potential.Best Pract Res Clin Rheumatol. 2025 Mar;39(1):102035. doi: 10.1016/j.berh.2025.102035. Epub 2025 Jan 25. Best Pract Res Clin Rheumatol. 2025. PMID: 39863438 Review.
-
Therapeutic potential of probiotics in gut microbial homeostasis and Rheumatoid arthritis.Int Immunopharmacol. 2024 Aug 20;137:112501. doi: 10.1016/j.intimp.2024.112501. Epub 2024 Jun 16. Int Immunopharmacol. 2024. PMID: 38885604 Review.
-
Molecular Mimicry Between Gut Microbiome and Rheumatoid Arthritis: Current Concepts.Med Sci (Basel). 2024 Dec 12;12(4):72. doi: 10.3390/medsci12040072. Med Sci (Basel). 2024. PMID: 39728421 Free PMC article. Review.
-
Gut microbiota in pre-clinical rheumatoid arthritis: From pathogenesis to preventing progression.J Autoimmun. 2023 Dec;141:103001. doi: 10.1016/j.jaut.2023.103001. Epub 2023 Mar 15. J Autoimmun. 2023. PMID: 36931952 Review.
Cited by
-
Progress on the mechanism of intestinal microbiota against colorectal cancer.Front Cell Infect Microbiol. 2025 Apr 28;15:1565103. doi: 10.3389/fcimb.2025.1565103. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40357397 Free PMC article. Review.
-
The Role of Methyl Canthin-6-one-2-carboxylate in Targeting the NLRP3 Inflammasome in Rheumatoid Arthritis Treatment.Curr Issues Mol Biol. 2025 Apr 7;47(4):254. doi: 10.3390/cimb47040254. Curr Issues Mol Biol. 2025. PMID: 40699653 Free PMC article. Review.
-
The gut-immune axis in primary immune thrombocytopenia (ITP): a paradigm shifts in treatment approaches.Front Immunol. 2025 Jun 12;16:1595977. doi: 10.3389/fimmu.2025.1595977. eCollection 2025. Front Immunol. 2025. PMID: 40574831 Free PMC article. Review.
-
Gut Microbes as the Major Drivers of Rheumatoid Arthritis: Our Microbes Are Our Fortune!Microorganisms. 2025 Jan 24;13(2):255. doi: 10.3390/microorganisms13020255. Microorganisms. 2025. PMID: 40005622 Free PMC article. Review.
-
Immunomodulatory properties of the gut microbiome: diagnostic and therapeutic potential for rheumatoid arthritis.Clin Exp Med. 2025 Jul 1;25(1):226. doi: 10.1007/s10238-025-01777-x. Clin Exp Med. 2025. PMID: 40591032 Free PMC article. Review.
References
-
- van der Woude D., Alemayehu W.G., Verduijn W., de Vries R.R., Houwing-Duistermaat J.J., Huizinga T.W., Toes R.E. Gene-environment interaction influences the reactivity of autoantibodies to citrullinated antigens in rheumatoid arthritis. Nat. Genet. 2010;42:814–816. doi: 10.1038/ng1010-814. - DOI - PubMed
-
- van der Helm-van Mil A.H.M., Wesoly J.Z., Huizinga T.W.J. Understanding the genetic contribution to rheumatoid arthritis. Curr. Opin. Rheumatol. 2005;17:299–304. doi: 10.1097/01.bor.0000160780.13012.be. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

