Erlotinib Improves the Response of Glioblastoma Cells Resistant to Photodynamic Therapy
- PMID: 39766391
- PMCID: PMC11674483
- DOI: 10.3390/brainsci14121192
Erlotinib Improves the Response of Glioblastoma Cells Resistant to Photodynamic Therapy
Abstract
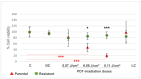
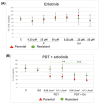
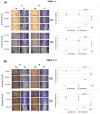
Background: Glioblastoma (GBM) is the most common and deadly type of brain cancer in adults. Dysregulation of receptor tyrosine kinase pathways, such as the epidermal growth factor receptor (EGFR), contributes to therapeutic resistance. Drugs that inhibit tyrosine kinase activity and monoclonal antibodies against EGFR are strategies used in clinical trials. Photodynamic therapy (PDT) is a tumor treatment that involves the administration of a photosensitizing drug, followed by its activation with visible light, which causes cell death due to oxidative stress. Although PDT helps prolong median survival in patients with GBM, complete remission has not been achieved. Populations of GBM cells have been obtained from the T98G line resistant to PDT with methyl-5-aminolevulinic acid (Me-ALA) for characterization, comparing them with the original parental population. Objective: The objective of this work was to evaluate the general response of T98G GBM cells resistant to PDT when EGFR activity is inhibited with the drug erlotinib. Methods and Results: It has been observed that the administration of the EGFR inhibitor drug in combination with PDT reduced viability (MTT) in resistant populations compared to PDT alone. Furthermore, the PpIX content (flow cytometry) was increased in the resistant population when cells were incubated with Me-ALA and erlotinib. Erlotinib prevented cell proliferation of parental and resistant spheroids. Wound closure was reduced in both parental and PDT-resistant populations. Conclusions: Our results indicate that EGFR activation would be relevant in the resistance of GBM cells to PDT.
Keywords: EGFR; erlotinib; glioblastoma; photodynamic therapy; resistance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
5-Aminolevulinic acid-mediated photodynamic therapy in combination with kinase inhibitor lapatinib enhances glioblastoma cell death.Apoptosis. 2024 Dec;29(11-12):1978-1987. doi: 10.1007/s10495-024-02012-w. Epub 2024 Aug 27. Apoptosis. 2024. PMID: 39190205 Free PMC article.
-
5-Aminolevulinic acid-mediated photodynamic therapy can target human glioma stem-like cells refractory to antineoplastic agents.Photodiagnosis Photodyn Ther. 2018 Dec;24:58-68. doi: 10.1016/j.pdpdt.2018.07.004. Epub 2018 Jul 7. Photodiagnosis Photodyn Ther. 2018. PMID: 29990642
-
Isolation and initial characterization of human glioblastoma cells resistant to photodynamic therapy.Photodiagnosis Photodyn Ther. 2021 Mar;33:102097. doi: 10.1016/j.pdpdt.2020.102097. Epub 2020 Nov 21. Photodiagnosis Photodyn Ther. 2021. PMID: 33232818
-
Photodynamic Therapy for Glioblastoma: Illuminating the Path toward Clinical Applicability.Cancers (Basel). 2023 Jun 30;15(13):3427. doi: 10.3390/cancers15133427. Cancers (Basel). 2023. PMID: 37444537 Free PMC article. Review.
-
5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas.J Neurooncol. 2019 Feb;141(3):595-607. doi: 10.1007/s11060-019-03103-4. Epub 2019 Jan 18. J Neurooncol. 2019. PMID: 30659522 Free PMC article. Review.
References
-
- Kiesel B., Wadiura L.I., Mischkulnig M., Makolli J., Sperl V., Borkovec M., Freund J., Lang A., Millesi M., Berghoff A.S., et al. Efficacy, Outcome, and Safety of Elderly Patients with Glioblastoma in the 5-ALA Era: Single Center Experience of More Than 10 Years. Cancers. 2021;13:6119. doi: 10.3390/cancers13236119. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

