Use of the MNCD Classification to Monitor Clinical Stage and Response to Levodopa-Entacapone-Carbidopa Intestinal Gel Infusion in Advanced Parkinson's Disease
- PMID: 39766443
- PMCID: PMC11674033
- DOI: 10.3390/brainsci14121244
Use of the MNCD Classification to Monitor Clinical Stage and Response to Levodopa-Entacapone-Carbidopa Intestinal Gel Infusion in Advanced Parkinson's Disease
Abstract
Background and objective: Staging Parkinson's disease (PD) with a novel simple classification called MNCD, based on four axes (Motor; Non-motor; Cognition; Dependency) and five stages, correlated with disease severity, patients' quality of life and caregivers' strain and burden. Our aim was to apply the MNCD classification in advanced PD patients treated with device-aided therapy (DAT).
Patients and methods: A multicenter observational retrospective study of the first patients to start the levodopa-entacapone-carbidopa intestinal gel (LECIG) in Spain was performed (LECIPARK study). The MNCD total score (from 0 to 12) and MNCD stages (from 1 to 5) were collected by the neurologist at V0 (before starting LECIG) and V2 (follow-up visit). Wilcoxon's signed rank and Marginal Homogeneity tests were applied to compare changes from V0 to V2.
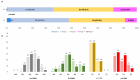
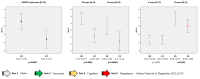
Results: Sixty-seven PD patients (58.2% males; 69.9 ± 9.3 years old) with a mean disease duration of 14.4 ± 6.5 years were included. The mean treatment duration (V2) was 172.9 ± 105.2 days. At V0, patients were classified as in stage 2 (35.8%), 3 (46.3%) or 4 (17.9%). The frequency of patients in stage 4 decreased to 9% at V2 (p = 0.001). The MNCD total score decreased from 6.27 ± 1.94 at V0 to 5.21 ± 2.23 (p < 0.0001). From V0 to V2, the motor (M; p < 0.0001) and non-motor symptom (N; p < 0.0001) burden decreased, and autonomy for the activities of daily living (D; p = 0.005) improved.
Conclusions: The MNCD classification could be useful to classify advanced PD patients and to monitor the response to a DAT.
Keywords: MNCD; advanced; device-aided therapy; non-motor symptoms; parkinson’s disease.
Conflict of interest statement
Diego Santos-García has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, Italfarmaco, Teva, Archímedes, Esteve, Stada, Merz, and grants from the Spanish Ministry of Economy and Competitiveness [PI16/01575] co-founded by ISCIII (Concesión de subvenciones de Proyectos de Investigación en Salud de la convocatoria 2020 de la Acción Estratégica en Salud 2017-2020 por el proyecto “PROGRESIÓN NO MOTORA E IMPACTO EN LA CALIDAD DE VIDA EN LA ENFERMEDAD DE PARKINSON”; Concesión de Contrato para la intensificación de la actividad investigadora en el Sistema Nacional de Salud, Convocatoria 2021, Instituto de Salud Carlos III). Lydia López-Manzanares compensated advisory services, consulting, research grant support, or speaker honoraria: AbbVie, Acorda, Bial, Intec Pharma, Italfarmaco, Pfizer, Roche, Teva, UCB, and Zambon. Inés Muro has received honoraria for lectures with educational purposes and advisory from ABBVIE, BIAL, Lundbeck, Zambon, and STADA. Pablo Lorenzo-Barreto received honoraria for educational presentations and advice service by Zambon and Orion Pharma. Elena Casas Peña has received honoraria for educational presentations and advice service by Bial. Rocío García-Ramos has received honoraria and grants for lecturing and advisory services from Abbvie, Zambón, Bial, Merk, and Stada. Tamara Fernández Valle: None. Carlos Morata-Martínez: None. Raquel Baviera-Muñoz is supported by a Rio Hortega contract (CM22/00099) from Instituto de Salud Carlos III. Irene Martínez-Torres has received honoraria from Abbvie, Bial, Ipsen, Medtronic, Merz, and Pallex for lecturing. María Álvarez-Sauco has received honoraria for educational presentations and advice services by Abbvie, UCB Pharma, Zambon, Bial, and Teva. Déborah Alonso-Modino: None. Inés Legarda has received honoraria for educational presentations and advice services by Abbvie, UCB Pharma, Zambon, Bial, and Teva. María Fuensanta Valero-García has received honoraria for educational presentations by Bial. José Andrés Suárez-Muñoz: None. Juan Carlos Martínez-Castrillo has received research support from Lundbeck, Italfarmaco, Allergan, Zambon, Merz, and Abbvie. He has received speaking honoraria from AbbVie, Bial, Italfarmaco, Lundbeck, Krka, TEVA, UCB, Zambon, Allergan, Ipsen, and Merz. Ana Belén Perona has received honoraria from Abbvie, Bial, Stada, and Merz for lecturing. Jose María Salom: None. Esther Cubo has received travel grants from Abbvie, Allergan, Boston, and lecturing honoraria from Abbvie, International Parkinson’s Disease Movement Disorder Society. Caridad Valero-Merino has received honoraria for educational services from Zambon, Abbvie, and UCB. Nuria López-Ariztegui compensated advisory services, consulting, research grant support, or speaker honoraria from Abbvie, Italfarmaco, Stada, Bial, Zambon, UCB, Lundbeck. Pilar Sánchez Alonso has received honoraria for educational presentations and advice services from Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva. Sabela Novo Ponte has received honoraria for educational presentations from Abbvie, Alter, and Schwabe Pharma Iberica and travel grants from Bial, Zambón, and Esteve. Elisa Gamo Gónzález has received honoraria for educational presentations and advice service by Italfarmaco, Bial, and Healthincode. Raquel Martín García has received honoraria for educational presentations and advice service by Almirall. Raúl Espinosa has received honoraria for educational presentations, advice service, and travel grants by AbbVie, Stada, Orion, Merz, Bial, Italfarmaco, Novartis, Pfizer, and Lundbeck. Mar Carmona has received honoraria from Abbvie, Bial, and Esteve and italfarmaco for lecturing and from Bial and Stada as consultant/scientific advisor. Cici Esmerali Feliz has received honoraria for educational presentations from Abbvie, Bial, Steve, Zambom, and Alter. Pedro García Ruíz has received personal compensation as a consultant/scientific advisory board from Italfarmaco, Britannia, Bial, Stada, and Esteve and speaking honoraria from Italfarmaco, Bial, Stada, and Esteve. Teresa Muñoz Ruíz: None. Beatriz Fernández Rodríguez: None. Marina Mata has recived honoraria for educational presentations or adviced services from Stada, Abbvie, Orion Pharma, Italfarmaco, Bial, Zambon, and Esteve.
Figures
References
-
- Santos García D., de Deus Fonticoba T., Cores C., Muñoz G., Paz González J.M., Martínez Miró C., Suárez E., Jesús S., Aguilar M., Pastor P., et al. Predictors of clinically significant quality of life impairment in Parkinson’s disease. NPJ Park. Dis. 2021;7:118. doi: 10.1038/s41531-021-00256-w. - DOI - PMC - PubMed
-
- Santos García D., Álvarez Sauco M., Calopa M., Carrillo F., Escamilla Sevilla F., Freire E., García Ramos R., Kulisevsky J., Gómez Esteban J.C., Legarda I., et al. MNCD: A New Tool for Classifying Parkinson’s Disease in Daily Clinical Practice. Diagnostics. 2021;12:55. doi: 10.3390/diagnostics12010055. - DOI - PMC - PubMed
-
- Santos-García D., Mir P., Cubo E., Vela L., Rodríguez-Oroz M.C., Martí M.J., Arbelo J.M., Infante J., Kulisevsky J., Martínez-Martín P., et al. COPPADIS-2015 (Cohort of Patients with Parkinson’s Disease in Spain, 2015), a global—Clinical evaluations, serum biomarkers, genetic studies and neuroimaging--prospective, multicenter, non-interventional, long-term study on Parkinson’s disease progression. BMC Neurol. 2016;16:26. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

