The Phenotype Changes of Astrocyte During Different Ischemia Conditions
- PMID: 39766455
- PMCID: PMC11674399
- DOI: 10.3390/brainsci14121256
The Phenotype Changes of Astrocyte During Different Ischemia Conditions
Abstract
Objectives: Dementia is becoming a major health problem in the world, and chronic brain ischemia is an established important risk factor in predisposing this disease. Astrocytes, as one major part of the blood-brain barrier (BBB), are activated during chronic cerebral blood flow hypoperfusion. Reactive astrocytes have been classified into phenotype pro-inflammatory type A1 or neuroprotective type A2. However, the specific subtype change of astrocyte and the mechanisms of chronic brain ischemia are still unknown.
Methods: In order to depict the phenotype changes and their possible roles during this process, a rat bilateral common carotid artery occlusion model (BCAO) was employed in the present study. Meanwhile, the signaling pathways that possibly regulate these changes were investigated as well.
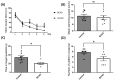
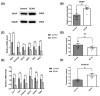
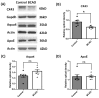
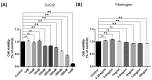
Results: After four-week occlusion, astrocytes in the cortex of BCAO rats were shown to be the A2 phenotype, identified by the significant up-regulation of S100a10 accompanied by the down-regulation of Connexin 43 (CX43) protein. Next, we established in vitro hypoxia models, which were set up by stimulating primary astrocyte cultures from rat cortex with cobalt chloride, low glucose, or/and fibrinogen. Consistent with in vivo data, the cultured astrocytes also transformed into the A2 phenotype with the up-regulation of S100a10 and the down-regulation of CX43. In order to explore the mechanism of CX43 protein changes, C6 astrocyte cells were handled in both hypoxia and low-glucose stimulus, in which decreased pERK and pJNK expression were found.
Conclusions: In conclusion, our data suggest that in chronic cerebral ischemia conditions, the gradual ischemic insults could promote the transformation of astrocytes into A2 type instead of A1 type, and the phosphorylation of CX43 was negatively regulated by the phosphorylation of ERK and JNK. Also, our data could provide some new evidence of how to leverage the endogenous astrocytes phenotype changes during CNS injury by promoting them to be "protector" and not "culprit".
Keywords: A2 phenotype; Connexin 43; astrocytes; dementia; ischemia.
Conflict of interest statement
There are no conflicts of interest among all the authors.
Figures





Similar articles
-
Transformation of A1/A2 Astrocytes Participates in Brain Ischemic Tolerance Induced by Cerebral Ischemic Preconditioning via Inhibiting NDRG2.Neurochem Res. 2024 Jul;49(7):1665-1676. doi: 10.1007/s11064-024-04134-8. Epub 2024 Feb 27. Neurochem Res. 2024. PMID: 38411782
-
The effects of A1/A2 astrocytes on oligodendrocyte linage cells against white matter injury under prolonged cerebral hypoperfusion.Glia. 2020 Sep;68(9):1910-1924. doi: 10.1002/glia.23814. Epub 2020 Feb 28. Glia. 2020. PMID: 32108971
-
Perioperative Dexmedetomidine attenuates brain ischemia reperfusion injury possibly via up-regulation of astrocyte Connexin 43.BMC Anesthesiol. 2020 Dec 7;20(1):299. doi: 10.1186/s12871-020-01211-7. BMC Anesthesiol. 2020. PMID: 33287729 Free PMC article.
-
Astrocyte subtype-specific approach to Alzheimer's disease treatment.Neurochem Int. 2021 May;145:104956. doi: 10.1016/j.neuint.2021.104956. Epub 2021 Jan 24. Neurochem Int. 2021. PMID: 33503465 Review.
-
Metabolic reprogramming and astrocytes polarization following ischemic stroke.Free Radic Biol Med. 2025 Feb 16;228:197-206. doi: 10.1016/j.freeradbiomed.2025.01.002. Epub 2025 Jan 3. Free Radic Biol Med. 2025. PMID: 39756488 Review.
Cited by
-
High-fat diet and chronic restraint stress exacerbate anxiety-depressive behaviors via astrocytic A1 phenotype transformation.Sci Rep. 2025 Apr 29;15(1):15031. doi: 10.1038/s41598-025-99355-4. Sci Rep. 2025. PMID: 40301496 Free PMC article.
References
-
- Rajeev V., Fann D.Y., Dinh Q.N., Kim H.A., De Silva T.M., Lai M.K.P., Chen C.L., Drummond G.R., Sobey C.G., Arumugam T.V. Pathophysiology of blood brain barrier dysfunction during chronic cerebral hypoperfusion in vascular cognitive impairment. Theranostics. 2022;12:1639–1658. doi: 10.7150/thno.68304. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

