Cell Wall-Mediated Antifungal Activity of the Aqueous Extract of Hedera helix L. Leaves Against Diplodia corticola
- PMID: 39766506
- PMCID: PMC11672613
- DOI: 10.3390/antibiotics13121116
Cell Wall-Mediated Antifungal Activity of the Aqueous Extract of Hedera helix L. Leaves Against Diplodia corticola
Abstract
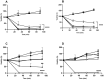
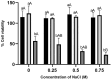
Background/Objectives: Cork oak forests have been declining due to fungal pathogens such as Diplodia corticola. However, the preventive fungicides against this fungus have restricted use due to the deleterious effects on human health and the environment, prompting the need for sustainable alternatives. Here, we describe the antifungal activity of an aqueous extract of Hedera helix L. leaves (HAE) against D. corticola and the possible mechanism of action. Results/Methods: The chemical analysis revealed compounds like the saponin hederacoside C, quinic acid, 5-O-caffeoylquinic acid, rutin, and glycoside derivatives of quercetin and kaempferol, all of which have been previously reported to possess antimicrobial activity. Remarkable in vitro antifungal activity was observed, reducing radial mycelial growth by 70% after 3 days of inoculation. Saccharomyces cerevisiae mutants, bck1 and mkk1/mkk2, affected the cell wall integrity signaling pathway were more resistant to HAE than the wild-type strain, suggesting that the extract targets kinases of the signaling pathway, which triggers toxicity. The viability under osmotic stress with 0.75 M NaCl was lower in the presence of HAE, suggesting the deficiency of osmotic protection by the cell wall. Conclusions: These results suggest that ivy extracts can be a source of new natural antifungal agents targeting the cell wall, opening the possibility of preventing fungal infections in cork oaks and improving the cork production sector using safer and more sustainable approaches.
Keywords: Diplodia corticola; Hedera helix water extract; Saccharomyces cerevisiae fungal model; anti-phytopathogen activity; antifungal mechanism of action.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Costa D., Tavares R.M., Baptista P., Lino-Neto T. The Influence of Endophytes on Cork Oak Forests Under a Changing Climate. In: Hodkinson T.R., Doohan F.M., Saunders M.J., Murphy B.R., editors. Endophytes for a Growing World. 1st ed. Cambridge University Press; Cambridge, UK: 2019. pp. 250–274.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

