Cloning and Functional Analysis of Skin Host Defense Peptides from Yakushima Tago's Brown Frog (Rana tagoi yakushimensis) and Development of Serum Endotoxin Detection System
- PMID: 39766517
- PMCID: PMC11672578
- DOI: 10.3390/antibiotics13121127
Cloning and Functional Analysis of Skin Host Defense Peptides from Yakushima Tago's Brown Frog (Rana tagoi yakushimensis) and Development of Serum Endotoxin Detection System
Abstract
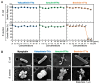
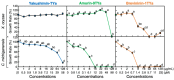
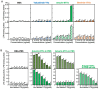
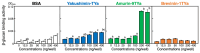
Background/Objective: Amphibian skin is a valuable source of host defense peptides (HDPs). This study aimed to identify HDPs with novel amino acid sequences from the skin of Rana tagoi yakushimensis and analyze their functions. Methods: cDNAs encoding HDP precursors were cloned and sequenced using RT-PCR and 3'-RACE. The novel HDPs were synthesized to evaluate their antimicrobial activity, antioxidant activity, and cytotoxicity. Antimicrobial activity was evaluated by way of broth microdilution and endotoxin- and β-glucan-binding capacity using an enzyme-linked endotoxin binding assay (ELEBA) and a modified ELEBA, respectively. Results: Nine cDNAs encoding precursors for various HDP families, including temporin, ranatuerin-2, brevinin-1, amurin-9, and a novel yakushimin peptide, were identified. Brevinin-1TYa exhibited antibacterial activity against Staphylococcus aureus, and brevinin-1TYa and amurin-9TYa induced morphological changes in Escherichia coli and S. aureus. Yakushimin-TYa, amurin-9TYa, and brevinin-1TYa showed concentration-dependent antibacterial effects against the plant pathogens Xanthomonas oryzae pv. oryzae and Clavibacter michiganensis subsp. michiganensis. Amurin-9TYa demonstrated strong binding affinity to lipopolysaccharide, lipoteichoic acid, and β-glucan, exhibited antioxidant activity, and lacked cytotoxicity, making it a promising therapeutic candidate. Moreover, brevinin-1TYa showed strong cytotoxicity, whereas yakushimin-TYa exhibited weak cytotoxicity. Conclusions: These findings highlight the potential of these peptides, particularly amurin-9TYa, for future applications as antimicrobial and therapeutic agents.
Keywords: ELEBA; ELGBA; Rana tagoi yakushimensis; amurin-9; anti-plant pathogenic activity; antioxidant activity; brevinin-1; frog skin; host defense peptides; yakushimin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- O’Neill J. The Review on Antimicrobial Resistance. Wellcome Trust and HM Government; London, UK: 2016. [(accessed on 8 November 2024)]. Tackling drug-resistant infections globally: Final report and recommendations; pp. 1–84. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20c....
-
- Popova K.B., Valsamatzi-Panagiotou A., Penchovsky R. New drug discovery strategies for targeting drug-resistant bacteria. Environ. Chem. Lett. 2021;19:1995–2004. doi: 10.1007/s10311-021-01181-3. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

