A Convenient One-Pot Synthesis of Novel Benzimidazole-Thiazinone Derivatives and Their Antimicrobial Activity
- PMID: 39766545
- PMCID: PMC11672583
- DOI: 10.3390/antibiotics13121155
A Convenient One-Pot Synthesis of Novel Benzimidazole-Thiazinone Derivatives and Their Antimicrobial Activity
Abstract
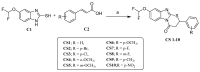
Background: The increasing prevalence of antimicrobial resistant highlights the urgent need for the new therapeutic agents. This study aimed to design and synthesize fused tricyclic benzimidazole-thiazinone derivatives (CS1-CS10) through a convenient method and evaluate their antimicrobial activity against various microorganisms. Methods: A series of fused tricyclic benzimidazole-thiazinone derivatives was rationally designed and synthesized in one pot by the reaction between trans substituted acrylic acids and 1H-benzo[d]imidazole-2-thiol using coupling reagent TBTU (2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate). The structure of these compounds was confirmed through various spectroscopic techniques like IR, 1H and 13C NMR, the DEPT and 2D-HMQC NMR techniques were also performed to confirm the relation of both carbon and proton. Further, the compounds were in vitro evaluated for their effectiveness against the Candida species and a panel of standard bacterial isolates. Results: The synthesized compounds showed moderate antimicrobial activity. Among all of the compounds, CS4 exhibited potent inhibition against Pseudomonas aeruginosa and Escherichia coli at 256 and 512 μg/mL concentrations, respectively. Additional research indicated that compound CS4 demonstrated a synergistic effect after combining with the standard antibacterial drug ciprofloxacin. Conclusions: These results suggest that CS4 is the best-synthesized antibacterial agent particularly in combination therapies. These findings highlight its promise for further development as a novel antibacterial agent.
Keywords: Candida species; TBTU; antibacterial; antifungal; synergetic effect; thiazinone.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- World Health Organization Global Report for Infectious Diseases of Poverty. World Health Organization; Geneva, Switzerland: 2012. pp. 1–168.
-
- CDC . Antibiotic Resistance Threats in the United States, 2019. Volume 10. U.S. Department of Health and Human Services, CDC; Atlanta, GA, USA: 2019. - DOI
-
- CDC . COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022. Volume 399. U.S. Department of Health and Human Services, CDC; Atlanta, GA, USA: 2022. pp. 629–655.
-
- Qadir T., Amin A., Sharma P.K., Jeelani I., Abe H. A Review on Medicinally Important Heterocyclic Compounds. Open Med. Chem. J. 2022;16:e187410452202280. doi: 10.2174/18741045-v16-e2202280. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

