Ellagic Acid Potentiates the Inhibitory Effects of Fluconazole Against Candida albicans
- PMID: 39766564
- PMCID: PMC11672414
- DOI: 10.3390/antibiotics13121174
Ellagic Acid Potentiates the Inhibitory Effects of Fluconazole Against Candida albicans
Abstract
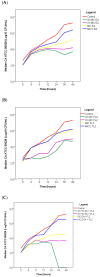
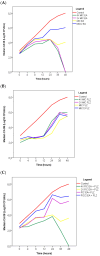
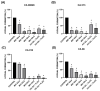
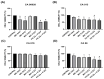
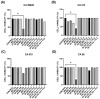
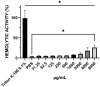
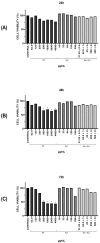
Background/Objectives: Antifungal resistance to azoles, coupled with the increasing prevalence of Candida albicans infections, represents a significant public health challenge and has driven the search for new natural compounds that can act as alternatives or adjuvants to the current antifungals. Ellagic acid (EA) has demonstrated antifungal activity; however, its effects are not fully understood. In this study, we investigated the in vitro anti-Candida activity of EA and its ability to potentiate the effects of fluconazole (FLZ) on C. albicans.Methods: The Minimum Inhibitory Concentration (MIC) of EA was determined by broth microdilution and its interaction with FLZ was assessed using a checkerboard assay. Additionally, we examined the effects of EA on yeast-to-hypha transition, inhibition of biofilm formation, time-kill kinetics, hemolytic activity, and cytotoxicity in HeLa ATCC® CCL-2™ cells. Results: EA exhibited MIC values ranging from 250 to 2000 µg/mL and showed synergistic and additive interactions with FLZ, resulting in a marked reduction in the MIC values of FLZ (up to 32-fold) and EA (up to 16-fold). In the time-kill assay, the most effective combinations were 4× EA MIC, 2× EA MIC, and FIC EA + FLZ, which showed fungicidal activity. Furthermore, EA did not show hemolytic activity and demonstrated low and dose-dependent cytotoxicity in HeLa cells, with no cytotoxic effects observed in combination with FLZ. EA and the synergistic combination of EA and FLZ interfered with both the yeast-to-hypha transition process in C. albicans cells and biofilm formation. In addition to its antifungal efficacy, EA demonstrated a favorable safety profile at the concentrations used. Conclusions: This study presents promising results regarding the potential use of EA in combination with FLZ for the treatment of C. albicans infections.
Keywords: anti-Candida activity; antibiofilm activity; ellagic acid; fluconazole.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- WHO . WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. World Health Organization; Geneva, Switzerland: 2022. p. 33.
-
- Giacobbe D.R., Esteves P., Bruzzi P., Mikulska M., Furfaro E., Mesini A., Tatarelli P., Grignolo S., Viscoli C., Colombo A.L., et al. Initial serum (1,3)-beta-D-glucan as a predictor of mortality in proven Candidaemia: Findings from a retrospective study in two teaching hospitals in Italy and Brazil. Clin. Microbiol. Infect. 2015;21:954.e9–954.e17. doi: 10.1016/j.cmi.2015.06.002. - DOI - PubMed
Grants and funding
- ACT- 05691/21/Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão
- UNIVERSAL-0965/19/Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão
- 317186/2023-0 and 305676/2019-9/National Council for Scientific and Technological Development
- Financial code 001/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
LinkOut - more resources
Full Text Sources

