Synthesis of Temporin-SHa Retro Analogs with Lysine Addition/Substitution and Antibiotic Conjugation to Enhance Antibacterial, Antifungal, and Anticancer Activities
- PMID: 39766603
- PMCID: PMC11672801
- DOI: 10.3390/antibiotics13121213
Synthesis of Temporin-SHa Retro Analogs with Lysine Addition/Substitution and Antibiotic Conjugation to Enhance Antibacterial, Antifungal, and Anticancer Activities
Abstract
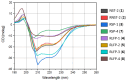
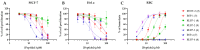
In the face of rising the threat of resistant pathogens, antimicrobial peptides (AMPs) offer a viable alternative to the current challenge due to their broad-spectrum activity. This study focuses on enhancing the efficacy of temporin-SHa derived NST-2 peptide (1), which is known for its antimicrobial and anticancer activities. We synthesized new analogs of 1 using three strategies, i.e., retro analog preparation, lysine addition/substitution, and levofloxacin conjugation. Analogs were tested in terms of their antibacterial, antifungal, and anticancer activities. Analog 2, corresponding to retro analog of NST-2, was found to be more active but also more hemolytic, reducing its selectivity index and therapeutic potential. The addition of lysine (in analog 3) and lysine substitution (in analog 7) reduced the hemolytic effect resulting in safer peptides. Conjugation with levofloxacin on the lysine side chain (in analogs 4 and 5) decreased the hemolytic effect but unfortunately also the antimicrobial and anticancer activities of the analogs. Oppositely, conjugation with levofloxacin at the N-terminus of the peptide via the β-alanine linker (in analogs 6 and 8) increased their antimicrobial and anticancer activity but also their hemolytic effect, resulting in less safe/selective analogs. In conclusion, lysine addition/substitution and levofloxacin conjugation, at least at the N-terminal position through the β-alanine linker, were found to enhance the therapeutic potential of retro analogs of NST-2 whereas other modifications decreased the activity or increased the toxicity of the peptides.
Keywords: AMPs; antibacterial; anticancer; antifungal; antimicrobial peptides; levofloxacin; temporin-SHa.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Synthesis of Second-Generation Analogs of Temporin-SHa Peptide Having Broad-Spectrum Antibacterial and Anticancer Effects.Antibiotics (Basel). 2024 Aug 11;13(8):758. doi: 10.3390/antibiotics13080758. Antibiotics (Basel). 2024. PMID: 39200058 Free PMC article.
-
Design, Synthesis and Characterization of [G10a]-Temporin SHa Dendrimers as Dual Inhibitors of Cancer and Pathogenic Microbes.Biomolecules. 2022 May 31;12(6):770. doi: 10.3390/biom12060770. Biomolecules. 2022. PMID: 35740895 Free PMC article.
-
Engineering of Antimicrobial Surfaces by Using Temporin Analogs to Tune the Biocidal/antiadhesive Effect.Molecules. 2019 Feb 24;24(4):814. doi: 10.3390/molecules24040814. Molecules. 2019. PMID: 30813478 Free PMC article.
-
Antimicrobial Peptide Analogs From Scorpions: Modifications and Structure-Activity.Front Mol Biosci. 2022 May 26;9:887763. doi: 10.3389/fmolb.2022.887763. eCollection 2022. Front Mol Biosci. 2022. PMID: 35712354 Free PMC article. Review.
-
Anticancer Potential of Antimicrobial Peptides: Focus on Buforins.Polymers (Basel). 2024 Mar 7;16(6):728. doi: 10.3390/polym16060728. Polymers (Basel). 2024. PMID: 38543342 Free PMC article. Review.
Cited by
-
Anticandidal Activity of Lipopeptides Containing an LL-37-Derived Peptide Fragment KR12.Molecules. 2025 Apr 3;30(7):1598. doi: 10.3390/molecules30071598. Molecules. 2025. PMID: 40286204 Free PMC article.
References
-
- Buommino E., Carotenuto A., Antignano I., Bellavita R., Casciaro B., Loffredo M.R., Merlino F., Novellino E., Mangoni M.L., Nocera F.P., et al. The Outcomes of Decorated Prolines in the Discovery of Antimicrobial Peptides from Temporin-L. ChemMedChem. 2019;14:1283–1290. doi: 10.1002/cmdc.201900221. - DOI - PubMed
-
- Bellavita R., Casciaro B., Di-Maro S., Brancaccio D., Carotenuto A., Falanga A., Cappiello F., Buommino E., Galdiero S., Novellino E., et al. First-in-class cyclic Temporin L analogue: Design, synthesis, and antimicrobial assessment. J. Med. Chem. 2021;64:11675–11694. doi: 10.1021/acs.jmedchem.1c01033. - DOI - PMC - PubMed
-
- Moreira Brito J.C., Carvalho L.R., De-Souza A.N., Carneiro G., Magalhaes P.P., Farias L.M., Guimaraes N.R., Verly R.M., Resende Jm De-Lima M.E. PEGylation of the antimicrobial peptide LyeTx Ib maintains structure-related biological properties and improves selectivity. Front. Mol. Biosci. 2022;9:1001508. doi: 10.3389/fmolb.2022.1001508. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

