Goondicones A-H: Spiro-Isoindolinone Heartworm Anthelmintics from an Australian Pasture-Soil-Derived Streptomyces sp
- PMID: 39766612
- PMCID: PMC11727212
- DOI: 10.3390/antibiotics13121222
Goondicones A-H: Spiro-Isoindolinone Heartworm Anthelmintics from an Australian Pasture-Soil-Derived Streptomyces sp
Abstract
Background/objectives: There is an urgent need for new and improved anthelmintics that are not constrained by existing resistance pathways and that can safeguard the health and welfare of animals.
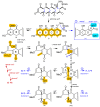
Methods: An integrated platform of chemical, bioassay, and cultivation profiling applied to a library of microbes isolated from Australian livestock pasture soil was used to detect and guide the production, isolation, characterization, identification, and evaluation of new natural products with anthelmintic properties.
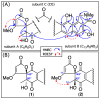
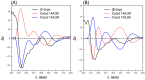
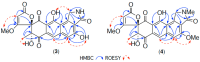
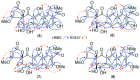
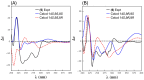
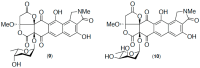
Results: A global natural products social (GNPS) molecular network analysis of 110 Australian pasture-soil-derived microbial extracts prioritized for antiparasitic activity identified unique molecular families in the extract of Streptomyces sp. S4S-00185A06, a strain selectively active against Dirofilaria immitis microfilariae. UPLC-DAD analysis identified metabolites with unique UV-vis chromophores and unprecedented molecular formulas. A chemical investigation of Streptomyces sp. S4S-00185A06 yielded goondicones A-H (1-8) as new examples of a rare class of spiro-isoindolinones, with structures assigned on the basis of detailed spectroscopic analysis, ECD calculations, and biosynthetic considerations.
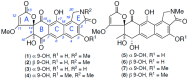
Conclusions: While goondicones 1-8 exhibit little to no in vitro inhibitory activity against Gram-positive, Gram-negative, and/or fungal pathogens, human carcinoma cells, or the livestock gastrointestinal parasite Haemonchus contortus L1-L3 larvae, 5 and 6 (and, to a lesser extent, 1) inhibit the motility of heartworm Dirofilaria immitis microfilaria (IC50 10-11 μM). A structure activity relationship analysis based on the co-metabolites 1-8 suggests that (i) an 8-OH is preferable to 8-oxo moiety, (ii) 20-NMe and 3-OH moieties are essential, and (iii) C-9 epimerization exerts no discernible impact on in vitro potency.
Keywords: Dirofilaria immitis; Soils for Science; Streptomyces; anthelmintic; citizen science; microbial natural product; spiro-isoindolinone.
Conflict of interest statement
David Bruhn, Cynthia Childs, and Yovany Moreno are employees of Boehringer Ingelheim Animal Health, USA Inc. All remaining authors declare no conflicts of interest.
Figures
Similar articles
-
Goondapyrones A-J: Polyketide α and γ Pyrone Anthelmintics from an Australian Soil-Derived Streptomyces sp.Antibiotics (Basel). 2024 Oct 18;13(10):989. doi: 10.3390/antibiotics13100989. Antibiotics (Basel). 2024. PMID: 39452255 Free PMC article.
-
Australian Marine and Terrestrial Streptomyces-Derived Surugamides, and Synthetic Analogs, and Their Ability to Inhibit Dirofilaria immitis (Heartworm) Motility.Mar Drugs. 2024 Jul 9;22(7):312. doi: 10.3390/md22070312. Mar Drugs. 2024. PMID: 39057421 Free PMC article.
-
Goondolinones A and B: Terpenyl-quinolin-4(1H)-ones from an Australian Volcanic Crater Soil-Derived Actinomadura sp., with Selective Activity against Dirofilaria immitis (Heartworm).J Nat Prod. 2024 Dec 27;87(12):2855-2862. doi: 10.1021/acs.jnatprod.4c01146. Epub 2024 Nov 24. J Nat Prod. 2024. PMID: 39582162
-
Glenthmycins A-M: Macrocyclic Spirotetronate Polyketide Antibacterials from the Australian Pasture Plant-Derived Streptomyces sp. CMB-PB041.J Nat Prod. 2022 Jun 24;85(6):1641-1657. doi: 10.1021/acs.jnatprod.2c00444. Epub 2022 May 31. J Nat Prod. 2022. PMID: 35640100 Review.
-
Advances in the discovery and development of anthelmintics by harnessing natural product scaffolds.Adv Parasitol. 2021;111:203-251. doi: 10.1016/bs.apar.2020.10.002. Epub 2021 Jan 8. Adv Parasitol. 2021. PMID: 33482975 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

