Rescue of Aberrant Splicing Caused by a Novel Complex Deep-intronic ABCA4 Allele
- PMID: 39766771
- PMCID: PMC11675205
- DOI: 10.3390/genes15121503
Rescue of Aberrant Splicing Caused by a Novel Complex Deep-intronic ABCA4 Allele
Abstract
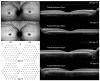
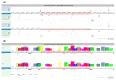
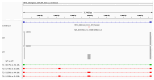
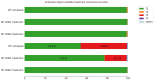
Background/Objectives: Stargardt disease (STGD1) is an autosomal recessive disorder caused by pathogenic variants in ABCA4 that affects the retina and is characterised by progressive central vision loss. The onset of disease manifestations varies from childhood to early adulthood. Methods: Whole exome (WES), whole gene, and whole genome sequencing (WGS) were performed for a patient with STGD1. Results: WES revealed a heterozygous pathogenic missense variant in ABCA4, but no second pathogenic variant was found. ABCA4 whole-gene sequencing, subsequent WGS, and segregation analysis identified a complex deep-intronic allele (NM_000350.2(ABCA4):c.[1555-5882C>A;1555-5784C>G]) in trans to the missense variant. Minigene assays combined with nanopore sequencing were performed to characterise this deep-intronic complex allele in more detail. Surprisingly, the reference minigene revealed the existence of two pseudoexons in intron 11 of the ABCA4 gene that are included in low-abundance (<1%) transcripts. Both pseudoexons could be confirmed in cDNA derived from wildtype retinal organoids. Despite mild splicing predictions, the variant minigene revealed that the complex deep-intronic allele substantially increased the abundance of transcripts that included the pseudoexon overlapping with the variants. Two antisense oligonucleotides (AONs) were designed to rescue the aberrant splicing events. Both AONs increased the proportion of correctly spliced transcripts, and one of them rescued correct splicing to reference levels. Conclusions: Minigene assays combined with nanopore sequencing proved instrumental in identifying low-abundance transcripts including pseudoexons from wildtype ABCA4 intron 11, one of which was substantially increased by the complex allele.
Keywords: ABCA4; Stargardt disease; antisense oligonucleotide; complex allele; deep-intronic variant; minigene; pseudoexon; rescue; retinal organoid; splicing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Fujinami K., Lois N., Davidson A.E., Mackay D.S., Hogg C.R., Stone E.M., Tsunoda K., Tsubota K., Bunce C., Robson A.G., et al. A Longitudinal Study of Stargardt Disease: Clinical and Electrophysiologic Assessment, Progression, and Genotype Correlations. Am. J. Ophthalmol. 2013;155:1075–1088.e13. doi: 10.1016/j.ajo.2013.01.018. - DOI - PubMed
-
- Strauss R.W., Ho A., Muñoz B., Cideciyan A.V., Sahel J.A., Sunness J.S., Birch D.G., Bernstein P.S., Michaelides M., Traboulsi E.I., et al. The Natural History of the Progression of Atrophy Secondary to Stargardt Disease (ProgStar) Studies: Design and Baseline Characteristics: Progstar Report No. 1. Ophthalmology. 2016;123:817–828. doi: 10.1016/j.ophtha.2015.12.009. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

