Potential Utility of Bacillus amyloliquefaciens SFB-1 as a Biocontrol Agent for Sweetpotato Black Rot Caused by Ceratocystis fimbriata
- PMID: 39766807
- PMCID: PMC11675987
- DOI: 10.3390/genes15121540
Potential Utility of Bacillus amyloliquefaciens SFB-1 as a Biocontrol Agent for Sweetpotato Black Rot Caused by Ceratocystis fimbriata
Abstract
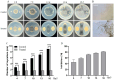
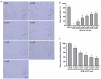
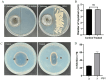
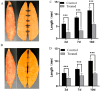
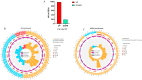
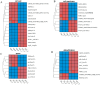
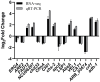
Background/Objectives: Sweetpotato black rot, caused by Ceratocystis fimbriata, is a severe fungal disease in sweetpotato production. Biological control strategies represent a promising, environmentally sustainable approach to managing this disease. This study investigates the biocontrol potential of Bacillus amyloliquefaciens SFB-1 against C. fimbriata. Methods: The antagonistic activities of strain SFB-1 on C. fimbriata were assessed through in vitro assays, including evaluations of mycelial inhibition, spore germination, and mycelial morphology. Pathogenicity assays on harvested sweetpotato roots assessed lesion diameter and depth. A transcriptomic analysis of C. fimbriata exposed to strain SFB-1 was performed to explore the underlying antifungal mechanism of SFB-1 on C. fimbriata. The qRT-PCR was employed to validate the RNA-seq results. Results: In vitro assays demonstrated that strain SFB-1 inhibited C. fimbriata mycelial growth by up to 81.01%, caused mycelial swelling, and completely suppressed spore germination at 108 CFU/mL. The cell-free supernatant of strain SFB-1 also suppressed C. fimbriata growth. Pathogenicity assays revealed that strain SFB-1 treatments reduced lesion diameter and depth on harvested sweetpotato roots by over 50% compared to untreated controls. Transcriptomic analysis of C. fimbriata treated with strain SFB-1 identified 1164 differentially expressed genes, with significant alterations in genes associated with cell wall integrity, cell membrane stability, spore germination, detoxification, and antioxidant responses. The qRT-PCR validation of 16 genes confirmed the consistency with the RNA-seq results. Conclusions: B. amyloliquefaciens SFB-1 demonstrates significant biocontrol efficacy against C. fimbriata through multiple mechanisms, positioning it as a promising solution for the sustainable management of sweetpotato black rot.
Keywords: Bacillus amyloliquefaciens; Ceratocystis fimbriata; biocontrol; sweetpotato; transcriptome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Alam M.K. A Comprehensive Review of Sweet Potato (Ipomoea batatas [L.] Lam): Revisiting the Associated Health Benefits. Trends Food Sci. Technol. 2021;115:512–529. doi: 10.1016/j.tifs.2021.07.001. - DOI
-
- Liu M., Meng Q., Wang S., Yang K., Tian J. Research Progress on Postharvest Sweet Potato Spoilage Fungi Ceratocystis fimbriata and Control Measures. Food Biosci. 2023;53:102627. doi: 10.1016/j.fbio.2023.102627. - DOI
-
- Mohsin S.M., Hasanuzzaman M., Parvin K., Morokuma M., Fujita M. Effect of Tebuconazole and Trifloxystrobin on Ceratocystis fimbriata to Control Black Rot of Sweet Potato: Processes of Reactive Oxygen Species Generation and Antioxidant Defense Responses. World J. Microbiol. Biotechnol. 2021;37:148. doi: 10.1007/s11274-021-03111-5. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

