Clinical and Cytogenetic Impact of Maternal Balanced Double Translocation: A Familial Case of 15q11.2 Microduplication and Microdeletion Syndromes with Genetic Counselling Implications
- PMID: 39766813
- PMCID: PMC11728287
- DOI: 10.3390/genes15121546
Clinical and Cytogenetic Impact of Maternal Balanced Double Translocation: A Familial Case of 15q11.2 Microduplication and Microdeletion Syndromes with Genetic Counselling Implications
Abstract
Background: Balanced chromosomal translocations occur in approximately 0.16 to 0.20% of live births. While most carriers are phenotypically normal, they are at risk of generating unbalanced gametes during meiosis, leading to genetic anomalies such as aneuploidies, deletions, duplications, and gene disruptions. These anomalies can result in spontaneous abortions or congenital anomalies, including neurodevelopmental disorders. Complex chromosomal rearrangements (CCRs) involving more than two chromosomes are rare but further increase the probability of producing unbalanced gametes. Neurodevelopmental disorders such as Angelman syndrome (AS) and duplication 15q11q13 syndrome (Dup15q) are associated with such chromosomal abnormalities.
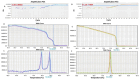
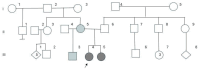
Methods: This study describes a family with a de novo maternal balanced double translocation involving chromosomes 13, 19, and 15, resulting in two offspring with unbalanced chromosomal abnormalities. Cytogenetic evaluations were performed using GTG banding, fluorescence in situ hybridization (FISH), and low-pass whole-genome sequencing (LP-WGS). Methylation analysis was conducted using methylation-sensitive high-resolution melting (MS-HRM) to diagnose Angelman syndrome.
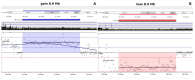
Results: The cytogenetic and molecular analyses identified an 8.9 Mb duplication in 15q11.2q13.3 in one child, and an 8.9 Mb deletion in the same region in the second child. Both abnormalities affected critical neurodevelopmental genes, such as SNRPN. FISH and MS-HRM confirmed the chromosomal imbalances and the diagnosis of Angelman syndrome in the second child. The maternal balanced translocation was found to be cryptic, contributing to the complex inheritance pattern.
Conclusion: This case highlights the importance of using multiple genetic platforms to uncover complex chromosomal rearrangements and their impact on neurodevelopmental disorders. The findings underscore the need for thorough genetic counseling, especially in families with such rare chromosomal alterations, to manage reproductive outcomes and neurodevelopmental risks.
Keywords: FISH; LP-WGS; double balanced translocation; genetic couseling.
Conflict of interest statement
Authors Carla Rosenberg, Patrícia Camacho Mazzonetto were employed by the company Diagnósticos da América S.A. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Peterson J.F., Geddes G.C., Basel D.G., Schippman D., Grignon J.W., Jr., vanTuinen P., Kappes U.P. Inheritance of a Balanced t(12;20)(q24.33;p12.2) and Unbalanced der(13)t(7;13)(p21.3;q33.2) from a Maternally Derived Double Balanced Translocation Carrier. J. Pediatr. Genet. 2018;7:35–39. - PMC - PubMed
-
- Wakeling E.L., Brioude F., Lokulo-sodipe O., O’Connell S.M., Salem J., bliek j Canton A.P., Chrzanowska K.H., Davies J.H., Dias R.P., Dubern B., et al. Diagnosis and management of silverrussell syndrome: First international consensus statement. Nat. Ver. Endocrinol. 2016;13:105–124. doi: 10.1038/nrendo.2016.138. - DOI - PubMed
-
- Mantovani G., Bastepe M., Monk D., De Sanctis L., Thiele S., Usardi A., Ahmed S.F., Bufo R., Choplin T., De Filippo G., et al. Diagnosis and management of pseudohypoparathyroidism and related disorders: First international consensus statement. Nat. Rev. Endocrinol. 2018;14:476–500. doi: 10.1038/s41574-018-0042-0. - DOI - PMC - PubMed
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

